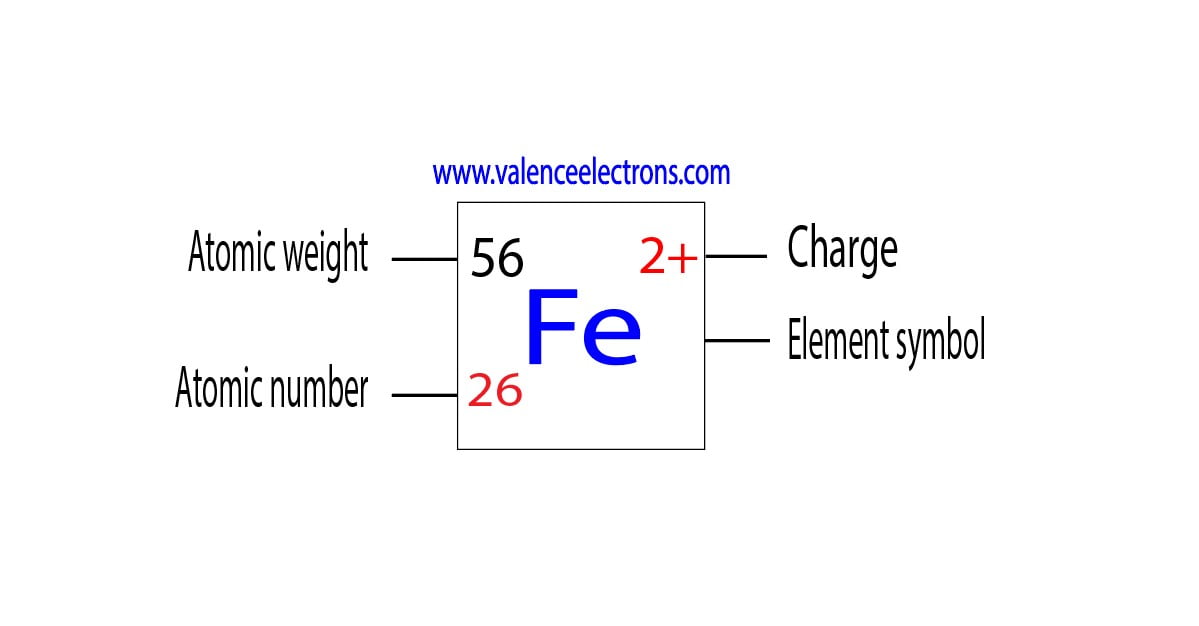
Does Iron Have Electrons . As a result, iron has eight valence electrons. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. Does iron have a full valence shell? Modern electron configurations now show iron as 3d6 4s2. Iron is a metal with atomic number 26 and 26 electrons. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. It describes how electrons are distributed among the various atomic. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals.
from valenceelectrons.com
Modern electron configurations now show iron as 3d6 4s2. Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. It describes how electrons are distributed among the various atomic. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Does iron have a full valence shell? As a result, iron has eight valence electrons. Iron is a metal with atomic number 26 and 26 electrons. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons).
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions)
Does Iron Have Electrons Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. As a result, iron has eight valence electrons. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. Iron is a metal with atomic number 26 and 26 electrons. Modern electron configurations now show iron as 3d6 4s2. Does iron have a full valence shell? Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). It describes how electrons are distributed among the various atomic.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Does Iron Have Electrons Modern electron configurations now show iron as 3d6 4s2. It describes how electrons are distributed among the various atomic. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are. Does Iron Have Electrons.
From rapidelectron.blogspot.com
Electron Configuration For An Atom Of Iron Rapid Electron Does Iron Have Electrons This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Modern electron configurations now show iron as 3d6. Does Iron Have Electrons.
From jacksofscience.com
Iron Valence Electrons Jacks Of Science Does Iron Have Electrons Modern electron configurations now show iron as 3d6 4s2. As a result, iron has eight valence electrons. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. Iron, which is the chief constituent of earth’s core, is the most abundant element in earth. Does Iron Have Electrons.
From sciencenotes.org
Iron Atom Science Notes and Projects Does Iron Have Electrons This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. Does iron have a full valence shell? Modern electron configurations now show iron as 3d6 4s2. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and. Does Iron Have Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Does Iron Have Electrons Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. Does iron have a full valence shell? As a result, iron has eight valence electrons. It describes how electrons are distributed among the various atomic. This is not the same as saying that 3d fills first because 3d is not full. Does Iron Have Electrons.
From www.periodictableprintable.com
Iron Periodic Table Electrons 2023 Periodic Table Printable Does Iron Have Electrons Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. It describes how electrons are distributed among the various atomic. In order to write the iron electron configuration we first need to know the number of. Does Iron Have Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Does Iron Have Electrons As a result, iron has eight valence electrons. It describes how electrons are distributed among the various atomic. Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Its electron configuration is [ar]. Does Iron Have Electrons.
From periodictable.me
How Many Valence Electrons Does Iron have Archives Dynamic Periodic Does Iron Have Electrons This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. It describes how electrons are distributed among the various atomic. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Iron, which is the chief. Does Iron Have Electrons.
From material-properties.org
Hierro Tabla periódica y propiedades atómicas Does Iron Have Electrons Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. It describes how electrons are distributed among the various atomic. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. Modern electron configurations now show iron as 3d6 4s2. Iron, which is the chief. Does Iron Have Electrons.
From www.alamy.com
Symbol and electron diagram for Iron illustration Stock Vector Image Does Iron Have Electrons As a result, iron has eight valence electrons. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. It describes how electrons are distributed among the various atomic. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states. Does Iron Have Electrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Does Iron Have Electrons The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Modern electron configurations now show iron as 3d6 4s2. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. Iron makes up 5 percent of earth’s crust. Does Iron Have Electrons.
From www.clipartmax.com
Diagram, Atom, Iron, Atomic, Bohr, Nucleus Many Valence Electrons Does Iron Have Electrons It describes how electrons are distributed among the various atomic. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. Does iron have a full valence shell? Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among. Does Iron Have Electrons.
From chemistry291.blogspot.com
【5 Steps】Electron Configuration of Iron(Fe) Electron configuration Does Iron Have Electrons Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. As a result, iron has eight valence electrons. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Modern electron configurations now show iron as 3d6 4s2. It describes how electrons are distributed. Does Iron Have Electrons.
From utedzz.blogspot.com
Periodic Table Iron Valence Electrons Periodic Table Timeline Does Iron Have Electrons This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. As a result, iron has eight valence electrons. Iron is a metal with atomic number 26 and 26 electrons. Iron makes up 5 percent of earth’s crust and is second in abundance to. Does Iron Have Electrons.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Does Iron Have Electrons Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. It describes how electrons are distributed among the various atomic. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. In order to write the iron electron configuration we first need to know the number of. Does Iron Have Electrons.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Does Iron Have Electrons Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Iron is a metal with atomic number 26 and 26 electrons. As a result, iron has eight. Does Iron Have Electrons.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Does Iron Have Electrons Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a whole. It describes how electrons are distributed among. Does Iron Have Electrons.
From jacksofscience.com
Iron Valence Electrons Jacks Of Science Does Iron Have Electrons As a result, iron has eight valence electrons. Iron is a metal with atomic number 26 and 26 electrons. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. Iron, which is the chief constituent of earth’s core, is the most abundant element. Does Iron Have Electrons.
From www.slideserve.com
PPT The Rusting of Iron PowerPoint Presentation, free download ID Does Iron Have Electrons This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). As a result, iron has eight valence electrons.. Does Iron Have Electrons.
From www.youtube.com
Valence Electrons for Fe (Iron) YouTube Does Iron Have Electrons Does iron have a full valence shell? It describes how electrons are distributed among the various atomic. Modern electron configurations now show iron as 3d6 4s2. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals.. Does Iron Have Electrons.
From valenceelectrons.com
How many protons, neutrons and electrons does iron have? Does Iron Have Electrons Modern electron configurations now show iron as 3d6 4s2. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). This is not the same as saying that. Does Iron Have Electrons.
From www.chegg.com
Solved ipoint How many valence electrons does iron have? 1 Does Iron Have Electrons It describes how electrons are distributed among the various atomic. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. As a result, iron has eight valence electrons. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum. Does Iron Have Electrons.
From www.vectorstock.com
Atom iron Royalty Free Vector Image VectorStock Does Iron Have Electrons Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the. Does Iron Have Electrons.
From www.dreamstime.com
Iron Atom Stock Illustrations 1,568 Iron Atom Stock Illustrations Does Iron Have Electrons Modern electron configurations now show iron as 3d6 4s2. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Its electron configuration is [ar] 3d6. Does Iron Have Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Iron (Fe Does Iron Have Electrons Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. It describes how electrons are distributed among the various atomic. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. In order to write the iron electron configuration we first need to know the number of. Does Iron Have Electrons.
From reviewhomedecor.co
Iron Periodic Table Electrons Review Home Decor Does Iron Have Electrons It describes how electrons are distributed among the various atomic. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. As a result, iron has. Does Iron Have Electrons.
From reviewhomedecor.co
Iron Periodic Table Protons Neutrons Electrons Review Home Decor Does Iron Have Electrons Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26. Does Iron Have Electrons.
From valenceelectrons.com
How Many Protons, Neutrons and Electrons Does Iron Have? Does Iron Have Electrons It describes how electrons are distributed among the various atomic. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Does iron have a full valence shell? As a result, iron has eight valence electrons. In order to write the iron electron configuration we first need to know the number of electrons. Does Iron Have Electrons.
From alquilercastilloshinchables.info
8 Photos Iron Periodic Table And Description Alqu Blog Does Iron Have Electrons Does iron have a full valence shell? As a result, iron has eight valence electrons. Iron is a metal with atomic number 26 and 26 electrons. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. Modern electron configurations now show iron as 3d6 4s2. The electron configuration of iron refers to. Does Iron Have Electrons.
From elchoroukhost.net
Iron Periodic Table Protons Neutrons And Electrons Elcho Table Does Iron Have Electrons Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. Does iron have a full valence shell? Modern electron configurations now show iron as 3d6 4s2. As a result, iron has eight valence electrons. Iron, which is the chief constituent of earth’s core, is the most abundant element in earth as a. Does Iron Have Electrons.
From makeup.vidalondon.net
What Is The Atomic Makeup Of Iron Makeup Vidalondon Does Iron Have Electrons Iron is a metal with atomic number 26 and 26 electrons. The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. As a result, iron has eight valence electrons. It describes how electrons are distributed among the various atomic. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum. Does Iron Have Electrons.
From valenceelectrons.com
How many valence electrons does iron(Fe) have? Does Iron Have Electrons Modern electron configurations now show iron as 3d6 4s2. Iron is a metal with atomic number 26 and 26 electrons. Its electron configuration is [ar] 3d6 4s2 and it can form compounds in oxidation states +2 and +3. This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d. Does Iron Have Electrons.
From www.toppr.com
Electronic Configuration of Iron Fe element Valency, Applications Does Iron Have Electrons The electron configuration of iron refers to the arrangement of electrons in the iron atom’s orbitals. As a result, iron has eight valence electrons. Iron makes up 5 percent of earth’s crust and is second in abundance to aluminum among the metals. It describes how electrons are distributed among the various atomic. Modern electron configurations now show iron as 3d6. Does Iron Have Electrons.
From www.youtube.com
How many valence electrons does iron have? YouTube Does Iron Have Electrons It describes how electrons are distributed among the various atomic. In order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there are 26 electrons). This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron. Does Iron Have Electrons.
From awesomehome.co
Iron Periodic Table Electrons Awesome Home Does Iron Have Electrons This is not the same as saying that 3d fills first because 3d is not full for iron, but the 6 3d electrons in iron are lower. As a result, iron has eight valence electrons. Modern electron configurations now show iron as 3d6 4s2. In order to write the iron electron configuration we first need to know the number of. Does Iron Have Electrons.