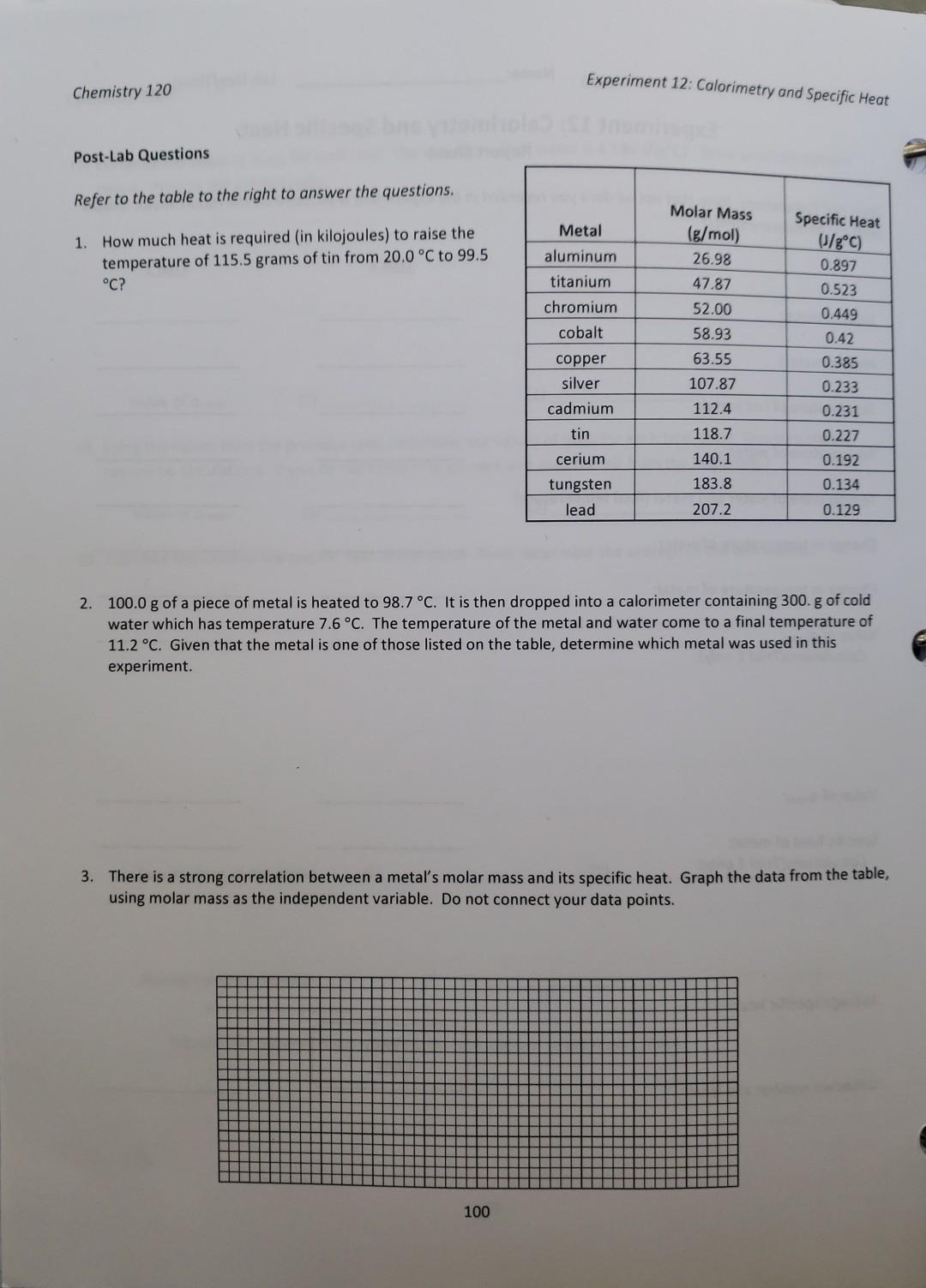
Lab Calorimetry And Specific Heat Edgenuity Answers . Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Calorimetry and specific heat lab part 1; Use a calculator, and round. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Calorimetry and specific heat purpose: Check all items that you used, either directly or indirectly, each time. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. You computed the specific heat for each of the four metals using the formula below:
from www.chegg.com
Calorimetry and specific heat purpose: Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. You computed the specific heat for each of the four metals using the formula below: Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Use a calculator, and round. Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Calorimetry and specific heat lab part 1; Check all items that you used, either directly or indirectly, each time. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal.
Solved Experiment 12 Calorimetry and Specific Heat
Lab Calorimetry And Specific Heat Edgenuity Answers Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Calorimetry and specific heat purpose: Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Calorimetry and specific heat lab part 1; Use a calculator, and round. Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. You computed the specific heat for each of the four metals using the formula below: Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Check all items that you used, either directly or indirectly, each time.
From www.pinterest.com
Calorimetry Lab Report Science diagrams, Thermodynamics, How to find out Lab Calorimetry And Specific Heat Edgenuity Answers Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in. Lab Calorimetry And Specific Heat Edgenuity Answers.
From brainly.com
please i need it Lab Calorimetry and Specific Heat Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat purpose: You computed the specific heat for each of the four metals using the formula below: Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Calorimetry. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Lab Calorimetry And Specific Heat Edgenuity Answers Check all items that you used, either directly or indirectly, each time. You computed the specific heat for each of the four metals using the formula below: Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Study with quizlet and memorize flashcards containing terms like beryllium. Lab Calorimetry And Specific Heat Edgenuity Answers.
From answerzoneschuster.z21.web.core.windows.net
Heat And Calorimetry Worksheet Answer Key Lab Calorimetry And Specific Heat Edgenuity Answers Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific. Lab Calorimetry And Specific Heat Edgenuity Answers.
From cityraven.com
👍 Heat effects and calorimetry lab answers. Heat Effects And Lab Calorimetry And Specific Heat Edgenuity Answers You computed the specific heat for each of the four metals using the formula below: Calorimetry and specific heat purpose: Use a calculator, and round. Check all items that you used, either directly or indirectly, each time. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal.. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Lab Calorimetry And Specific Heat Edgenuity Answers Check all items that you used, either directly or indirectly, each time. You computed the specific heat for each of the four metals using the formula below: Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Calorimetry and specific heat purpose: Calorimetry and specific heat lab part 1; Study with quizlet and memorize. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.transtutors.com
(Get Answer) PreLab Questions Calorimetry And Specific Heat Of Metal Lab Calorimetry And Specific Heat Edgenuity Answers Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Check all items that you used, either directly or indirectly, each time. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Use your data, the equation below, and the specific. Lab Calorimetry And Specific Heat Edgenuity Answers.
From studylib.net
Calorimetry Lab Specific Heat Capacity Lab Calorimetry And Specific Heat Edgenuity Answers Use a calculator, and round. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Calorimetry and specific heat purpose: Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Calorimetry and specific heat lab part 1; You computed the specific. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab calorimetry and specific heat student Lab Calorimetry And Specific Heat Edgenuity Answers Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Use a calculator, and round. You computed the specific heat for each of the four metals using the formula below: Calorimetry and. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat purpose: Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. You computed the specific heat for each of the four metals using the formula below: Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studypool.com
SOLUTION Lab calolimether and specific heat Studypool Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat purpose: Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. You computed the specific heat for each of the. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.englishworksheet.my.id
Specific Heat Worksheet Answers Lab Calorimetry And Specific Heat Edgenuity Answers You computed the specific heat for each of the four metals using the formula below: Calorimetry and specific heat lab part 1; Use a calculator, and round. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Use your data, the equation below, and the specific heat of. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.chegg.com
Solved Lab 5 Report Calorimetry Determination of Specific Lab Calorimetry And Specific Heat Edgenuity Answers Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Study with. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Lab Calorimetry And Specific Heat Edgenuity Answers Use a calculator, and round. You computed the specific heat for each of the four metals using the formula below: Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Use your. Lab Calorimetry And Specific Heat Edgenuity Answers.
From brainly.com
Does anyone have the table for the Calorimetry and Specific Heat lab Lab Calorimetry And Specific Heat Edgenuity Answers Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Calorimetry and specific heat purpose: Check all items that you used, either directly or indirectly, each time. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.chegg.com
Solved Calorimetry and Specific Heat MATERIALS AND EQUTPMENT Lab Calorimetry And Specific Heat Edgenuity Answers You computed the specific heat for each of the four metals using the formula below: Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight.. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studypool.com
SOLUTION Lab calorimetry and specific heat test 2023 Studypool Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat lab part 1; Check all items that you used, either directly or indirectly, each time. Use a calculator, and round. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Calorimetry and specific heat purpose: You computed the specific heat for each of. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab calorimetry and specific heat student Lab Calorimetry And Specific Heat Edgenuity Answers Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Use a calculator, and round. Explore how the specific heat of a substance can be determined using a. Lab Calorimetry And Specific Heat Edgenuity Answers.
From manualfixfaber.z19.web.core.windows.net
Calculating Specific Heat Worksheet Answers Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat lab part 1; Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Use your data, the equation below, and the specific heat of. Lab Calorimetry And Specific Heat Edgenuity Answers.
From browsegrades.net
Student Exploration Calorimetry Lab Vocabulary calorie, calorimeter Lab Calorimetry And Specific Heat Edgenuity Answers Use a calculator, and round. You computed the specific heat for each of the four metals using the formula below: Calorimetry and specific heat lab part 1; Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Use your data, the equation below, and the specific heat. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studocu.com
3210 07 04 student guide Copyright © Edgenuity Inc. Lab Calorimetry Lab Calorimetry And Specific Heat Edgenuity Answers Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Check all items that you used, either directly or indirectly, each time. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Use your data, the equation below,. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.chegg.com
Laboratory 9 CALORIMETRY SPECIFIC HEAT OF A METAL Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat lab part 1; Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Study with quizlet and memorize flashcards containing. Lab Calorimetry And Specific Heat Edgenuity Answers.
From studylibfreytag.z21.web.core.windows.net
Calorimetry Problems Worksheet Answers Lab Calorimetry And Specific Heat Edgenuity Answers Check all items that you used, either directly or indirectly, each time. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Use a calculator, and round. Study with quizlet and memorize flashcards containing terms. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.scribd.com
Lab 07Specific Heat & Calorimetry PDF PDF Heat Temperature Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat lab part 1; You computed the specific heat for each of the four metals using the formula below: Check all items that you used, either directly or indirectly, each time. Calorimetry and specific heat purpose: Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Lab Calorimetry And Specific Heat Edgenuity Answers Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. You computed the specific heat for each of the four metals using the formula below: Use a calculator,. Lab Calorimetry And Specific Heat Edgenuity Answers.
From kimora-kbowen.blogspot.com
Temperature and Specific Heat Lab 4 Answers Lab Calorimetry And Specific Heat Edgenuity Answers Use a calculator, and round. You computed the specific heat for each of the four metals using the formula below: Calorimetry and specific heat purpose: Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Check all items that you used, either directly or indirectly, each time. Use your data, the. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studocu.com
Calorimetry and Specific Heat Lab Question Using a calorimeter, how Lab Calorimetry And Specific Heat Edgenuity Answers Use a calculator, and round. Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong,. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.scribd.com
Activity 4 Calorimetry and Specific Heat Answer Sheet PDF Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat lab part 1; Check all items that you used, either directly or indirectly, each time. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each metal. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Calorimetry and. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.chegg.com
Solved Experiment 12 Calorimetry and Specific Heat Lab Calorimetry And Specific Heat Edgenuity Answers Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Calorimetry and specific heat purpose: Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Calorimetry and specific heat lab part 1; Study with quizlet. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.chegg.com
Solved Experiment 25 Report Sheet Date A. Specific Heat of a Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat lab part 1; Calorimetry and specific heat purpose: Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. You computed the specific heat for each of the four metals using the formula below: Use your data, the equation below, and the specific heat of. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.scribd.com
Calorimetry Lab SE PDF Calorie Heat Capacity Lab Calorimetry And Specific Heat Edgenuity Answers Calorimetry and specific heat lab part 1; Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Check all items that you used, either directly or indirectly, each time. Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific. Lab Calorimetry And Specific Heat Edgenuity Answers.
From wordworksheet.com
Specific Heat Worksheet Answers Lab Calorimetry And Specific Heat Edgenuity Answers Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studypool.com
SOLUTION Gizmo student exploration calorimetry lab answer key Studypool Lab Calorimetry And Specific Heat Edgenuity Answers Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Calorimetry and specific heat lab part 1; Use your data, the equation below, and the specific heat of water (4 j/g°c) to compute the specific heat values of each metal. Check all items that you used, either directly or indirectly, each time. Calorimetry and. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.studocu.com
Copy of Lab 5 Calorimetry and Specific Heat Lab Calorimetry and Lab Calorimetry And Specific Heat Edgenuity Answers Check all items that you used, either directly or indirectly, each time. Explore how the specific heat of a substance can be determined using a “coffee cup” calorimeter. Study with quizlet and memorize flashcards containing terms like beryllium is a rare metal that is gray in color, strong, and lightweight. Calorimetry and specific heat purpose: Use your data, the equation. Lab Calorimetry And Specific Heat Edgenuity Answers.
From www.chegg.com
Solved REPORT FOR EXPERIMENT5 Calorimetry and Specific Heat Lab Calorimetry And Specific Heat Edgenuity Answers Study with quizlet and memorize flashcards containing terms like heat, two factors of temperature change., specific heat capacity and. Check all items that you used, either directly or indirectly, each time. Calorimetry and specific heat lab part 1; Use your data, the equation below, and the specific heat of water (4.184 j/g0c) to compute the specific heat values of each. Lab Calorimetry And Specific Heat Edgenuity Answers.