Zinc Iron Formula . With the middling atomic number 30,. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zinc can be readily cast or. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide.
from www.teachoo.com
Zinc can be readily cast or. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. With the middling atomic number 30,. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide.
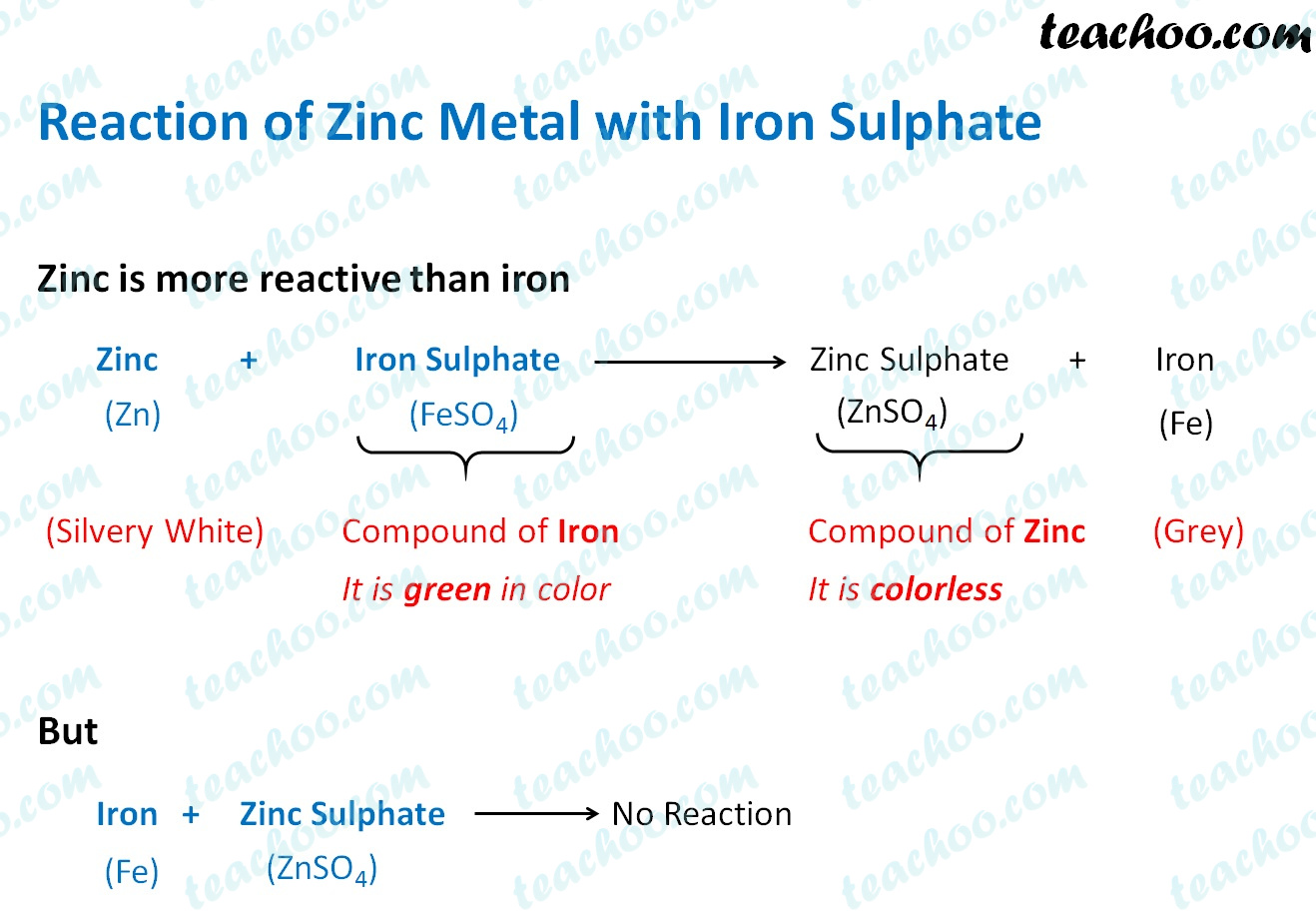
Displacement Reaction and Reactivity Series Concepts
Zinc Iron Formula Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc can be readily cast or. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. With the middling atomic number 30,. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals.
From www.dreamstime.com
Chemical Element Zinc from the Periodic Table Stock Vector Zinc Iron Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. With the middling atomic number 30,. The formula for an ionic compound. Zinc Iron Formula.
From www.alamy.es
Óxido de zinc, molécula de ZnO. Es compuesto ingrediente Zinc Iron Formula Zinc can be readily cast or. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zn + feo = fe + zno is a single displacement (substitution) reaction where one. Zinc Iron Formula.
From de.dreamstime.com
Zinkglycinat Ist Eine Molekulare Chemische Formel ZincInfografiken Zinc Iron Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. With the middling atomic number 30,. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zn + feo = fe + zno is a single displacement (substitution) reaction where one. Zinc Iron Formula.
From www.alamy.com
Zinc Periodic Table of the Elements Vector illustration eps 10 Stock Zinc Iron Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zn + feo = fe +. Zinc Iron Formula.
From www.planetnatural.com
Iron & Zinc Fertilizer by Liquinox Natural Zinc Iron Formula The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. With the middling atomic. Zinc Iron Formula.
From armsingle10.pythonanywhere.com
Peerless Molecular Formula Of Rust Write The Balanced Equation Zinc Iron Formula Zinc can be readily cast or. With the middling atomic number 30,. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. The formula for an ionic compound must give the same number. Zinc Iron Formula.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts Zinc Iron Formula Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc can. Zinc Iron Formula.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Iron Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. With the middling atomic number 30,. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. The formula for an. Zinc Iron Formula.
From www.alamy.com
Zinc glycinate is a molecular chemical formula. Zinc infographics Zinc Iron Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zinc can be readily cast or. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes. Zinc Iron Formula.
From projectopenletter.com
Zinc Sulfate And Iron Ii Bromide Precipitate Printable Form Zinc Iron Formula With the middling atomic number 30,. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Represented in the periodic table as zn, zinc is a transition metal,. Zinc Iron Formula.
From cartoondealer.com
Zinc Sulfate Is A Molecular Chemical Formula. Zinc Infographics. Vector Zinc Iron Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc can be readily cast or. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one. Zinc Iron Formula.
From www.youtube.com
Balancing the Equation Zn + FeCl3 = ZnCl2 + Fe (and Type of Reaction Zinc Iron Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc can be readily. Zinc Iron Formula.
From www.alamy.com
Symbol and electron diagram for Zinc Stock Vector Image & Art Alamy Zinc Iron Formula With the middling atomic number 30,. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. The lighter group 3a. Zinc Iron Formula.
From de.dreamstime.com
Zinkcitrat Ist Eine Molekulare Chemische Formel ZincInfografiken Zinc Iron Formula Zinc can be readily cast or. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. With the. Zinc Iron Formula.
From www.vectorstock.com
Zinc chemical element Royalty Free Vector Image Zinc Iron Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. With the middling atomic number 30,. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Zn + feo = fe +. Zinc Iron Formula.
From blog.thepipingmart.com
Which metal is more reactive iron or zinc Zinc Iron Formula Zinc can be readily cast or. With the middling atomic number 30,. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. Represented in the periodic table as zn,. Zinc Iron Formula.
From www.alamy.com
Zinc sulfate is a molecular chemical formula. Zinc infographics. Vector Zinc Iron Formula Zinc can be readily cast or. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. The formula for an ionic compound must give the same number of positive. Zinc Iron Formula.
From www.dreamstime.com
Zinc Periodic Table of the Elements Vector Illustration Stock Vector Zinc Iron Formula With the middling atomic number 30,. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The formula for an ionic compound must contain the same number of positive. Zinc Iron Formula.
From www.alamy.com
Zinc oxide is a molecular chemical formula. Zinc infographics. Vector Zinc Iron Formula Zinc can be readily cast or. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The formula. Zinc Iron Formula.
From www.alamy.es
Deficiencia de zinc Imágenes de stock en blanco y negro Alamy Zinc Iron Formula Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The formula for an ionic compound must give the same number of positive and negative charges close. Zinc Iron Formula.
From www.alamy.es
El zincsulfatado es una fórmula química molecular. Infografías sobre Zinc Iron Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a. The formula for an ionic compound. Zinc Iron Formula.
From www.dreamstime.com
Chemical Formula of Iron Over Black Background Stock Footage Video of Zinc Iron Formula Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). With the middling atomic number 30,. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. Represented in the periodic table as zn, zinc is a. Zinc Iron Formula.
From www.britannica.com
zinc Properties, Uses, & Facts Britannica Zinc Iron Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and. With the middling atomic number 30,. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter that causes a.. Zinc Iron Formula.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Iron Formula Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zinc can be readily cast or. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). With the middling atomic number 30,.. Zinc Iron Formula.
From cartoondealer.com
Zinc Zn Chemical Element Periodic Table Stock Photo CartoonDealer Zinc Iron Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Zinc can be readily cast or. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The formula for an ionic compound must give the same number of positive. Zinc Iron Formula.
From www.fishersci.com
Zinc iron oxide, Thermo Scientific Chemicals, Quantity 25 g Fisher Zinc Iron Formula With the middling atomic number 30,. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. The formula for an ionic compound must contain the. Zinc Iron Formula.
From de.dreamstime.com
Zinkcitrat Ist Eine Molekulare Chemische Formel ZincInfografiken Zinc Iron Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. With the middling atomic number 30,. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). Represented in the periodic table as zn, zinc. Zinc Iron Formula.
From zinc.ca
Zinc and iron deficiency Zinc.ca Zinc Iron Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Zinc can be readily cast or. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. The formula for an ionic compound must give the same number of positive and negative charges close. Zinc Iron Formula.
From material-properties.org
Iron and Zinc Comparison Properties Material Properties Zinc Iron Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron (galvanizing). With the middling atomic number 30,. The formula for an ionic compound must give the same number of positive and negative charges close charge property of matter. Zinc Iron Formula.
From www.vectorstock.com
Zinc acetate is a molecular chemical formula zinc Vector Image Zinc Iron Formula The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one mole of iron (ii) oxide. Zinc can be readily cast or. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals. Zinc Iron Formula.
From www.alamy.es
La monometionina de zinc es una fórmula química molecular. Infografías Zinc Iron Formula The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. With the middling atomic number 30,. Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zn +. Zinc Iron Formula.
From www.youtube.com
How to Write the Formula for Zinc phosphate YouTube Zinc Iron Formula With the middling atomic number 30,. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium and mercury. Zinc can be readily cast or. Zinc and cadmium (group 2b) form [+2]. Zinc Iron Formula.
From www.youtube.com
Net Ionic Equation for Zn + CuSO4 Zinc + Copper (II) Sulfate YouTube Zinc Iron Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Zinc can be readily cast or. Represented in the periodic table as zn, zinc is a transition metal, grouped with cadmium. Zinc Iron Formula.
From www.dreamstime.com
Zinc Monomethionine is a Molecular Chemical Formula. Zinc Infographics Zinc Iron Formula Zinc and cadmium (group 2b) form [+2] cations like the group 1b alkaline earth metals. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. Zn + feo = fe + zno is a single displacement (substitution) reaction where one mole of zinc [zn] and one. Zinc Iron Formula.
From es.dreamstime.com
El Sulfato De Zinc Es Una Fórmula Química Molecular. Infografía De Zinc Zinc Iron Formula With the middling atomic number 30,. The formula for an ionic compound must contain the same number of positive and negative charges close charge property of matter that causes a. The lighter group 3a metals (aluminum, galium and indium), along with scandium and. Zinc is reasonably resistant to corrosion and is used as a covering for baser metals like iron. Zinc Iron Formula.