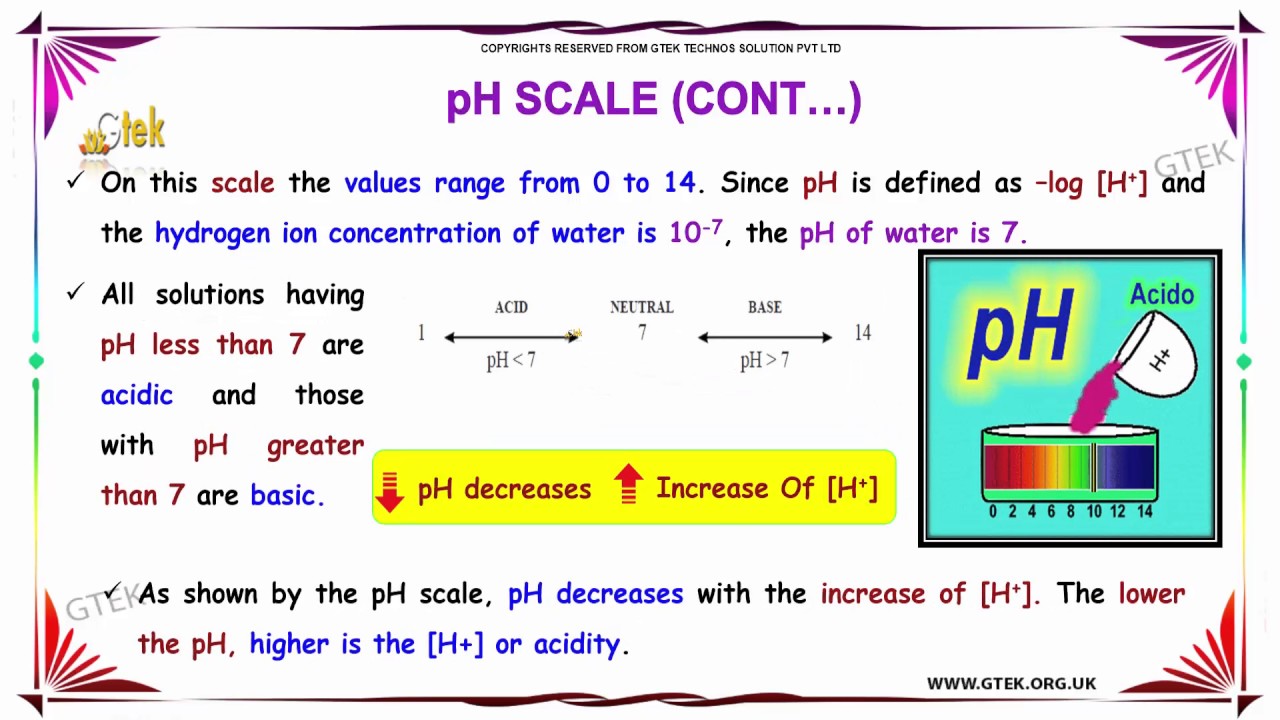
What Does Ph Mean In Electrical Terms . — as discussed, ph is the measurement of a specific ion (i.e., hydrogen). the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). — ph is defined as the negative logarithm of the concentration of h+ ions. Chemical solutions with ph levels below zero and above 14. The cell consists of an indicating electrode whose potential. The starting point is an electrochemical cell. As a result, the meaning of the name is justified as hydrogen power. Some react violently, others moderately, and still others do not. Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. We know that not all acids and bases react at the same rate with the same chemical compound.
from www.youtube.com
— as discussed, ph is the measurement of a specific ion (i.e., hydrogen). We know that not all acids and bases react at the same rate with the same chemical compound. — ph is defined as the negative logarithm of the concentration of h+ ions. Chemical solutions with ph levels below zero and above 14. Some react violently, others moderately, and still others do not. As a result, the meaning of the name is justified as hydrogen power. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). The cell consists of an indicating electrode whose potential. The starting point is an electrochemical cell.
ph scale and relation between ph and poh electrochemistry class 12
What Does Ph Mean In Electrical Terms The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. Chemical solutions with ph levels below zero and above 14. — ph is defined as the negative logarithm of the concentration of h+ ions. As a result, the meaning of the name is justified as hydrogen power. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. The starting point is an electrochemical cell. Some react violently, others moderately, and still others do not. The cell consists of an indicating electrode whose potential. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). — as discussed, ph is the measurement of a specific ion (i.e., hydrogen). We know that not all acids and bases react at the same rate with the same chemical compound.
From www.thoughtco.com
pH Definition and Equation in Chemistry What Does Ph Mean In Electrical Terms We know that not all acids and bases react at the same rate with the same chemical compound. Chemical solutions with ph levels below zero and above 14. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14. What Does Ph Mean In Electrical Terms.
From thechemistrynotes.com
pH Definition, Calculation, and Significance What Does Ph Mean In Electrical Terms — ph is defined as the negative logarithm of the concentration of h+ ions. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. We know that not all acids and bases react at the same rate with the same chemical compound. the basic ph scale extends from. What Does Ph Mean In Electrical Terms.
From byjus.com
pH Of Acids And Bases Calculate pH Value Chemistry Byju's What Does Ph Mean In Electrical Terms Some react violently, others moderately, and still others do not. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. — ph is defined as the negative logarithm of the. What Does Ph Mean In Electrical Terms.
From www.britannica.com
PH Definition, Uses, & Facts Britannica What Does Ph Mean In Electrical Terms We know that not all acids and bases react at the same rate with the same chemical compound. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). — ph is defined as the negative logarithm of the concentration of h+ ions. — the potential of hydrogen, or ph,. What Does Ph Mean In Electrical Terms.
From mavink.com
Ph Scale Diagram What Does Ph Mean In Electrical Terms The starting point is an electrochemical cell. Some react violently, others moderately, and still others do not. We know that not all acids and bases react at the same rate with the same chemical compound. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). — the potential of hydrogen,. What Does Ph Mean In Electrical Terms.
From www.alamy.com
Diagram of the pH scale with examples of acidic, neutral and alkaline What Does Ph Mean In Electrical Terms Chemical solutions with ph levels below zero and above 14. As a result, the meaning of the name is justified as hydrogen power. We know that not all acids and bases react at the same rate with the same chemical compound. — ph is defined as the negative logarithm of the concentration of h+ ions. the basic ph. What Does Ph Mean In Electrical Terms.
From www.sliderbase.com
PH Scale and Calculations Presentation Chemistry What Does Ph Mean In Electrical Terms — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Chemical solutions with ph levels below zero and above 14. — ph is defined as the negative logarithm of the concentration of h+ ions. — as discussed, ph is the measurement of a specific ion (i.e., hydrogen). The. What Does Ph Mean In Electrical Terms.
From www.slideserve.com
PPT What is pH? PowerPoint Presentation, free download ID5570624 What Does Ph Mean In Electrical Terms — as discussed, ph is the measurement of a specific ion (i.e., hydrogen). — ph is defined as the negative logarithm of the concentration of h+ ions. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to. What Does Ph Mean In Electrical Terms.
From www.clemson.edu
What is pH? Extension Clemson University South Carolina What Does Ph Mean In Electrical Terms Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. — ph is defined as the negative logarithm of the concentration of h+ ions. We know that not all acids and bases react at the same rate with the same chemical compound.. What Does Ph Mean In Electrical Terms.
From forestchemistry.weebly.com
Unit 5 Major Classes of Reactions Mrs. Forest's Chemistry Class site What Does Ph Mean In Electrical Terms Some react violently, others moderately, and still others do not. Chemical solutions with ph levels below zero and above 14. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). We know that not all acids and bases react at the same rate with the same chemical compound. As a result,. What Does Ph Mean In Electrical Terms.
From sciencetrends.com
What Does pH Stand For And Mean? Science Trends What Does Ph Mean In Electrical Terms We know that not all acids and bases react at the same rate with the same chemical compound. Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. — the potential of hydrogen, or ph, is a measure of the concentration of. What Does Ph Mean In Electrical Terms.
From www.waterfiltermag.com
What is pH? Definition and Measurement Water Filter Mag What Does Ph Mean In Electrical Terms Chemical solutions with ph levels below zero and above 14. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Some react violently, others moderately, and still others do not. The starting point is an electrochemical cell. the basic ph scale extends from 0 (strong acid) to 7 (neutral,. What Does Ph Mean In Electrical Terms.
From www.sciencelearn.org.nz
pH scale — Science Learning Hub What Does Ph Mean In Electrical Terms The cell consists of an indicating electrode whose potential. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. — as discussed, ph is the measurement of a specific ion (i.e., hydrogen). Some react. What Does Ph Mean In Electrical Terms.
From www.mometrix.com
pH Overview (Chemistry Review Video) What Does Ph Mean In Electrical Terms The cell consists of an indicating electrode whose potential. As a result, the meaning of the name is justified as hydrogen power. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Ph is an abbreviation for power of hydrogen where p is short for the german word for power,. What Does Ph Mean In Electrical Terms.
From www.slideserve.com
PPT Biology I What is pH? PowerPoint Presentation, free download ID What Does Ph Mean In Electrical Terms The cell consists of an indicating electrode whose potential. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). — ph is defined as the negative logarithm of the concentration of h+ ions. We know that not all acids and bases react at the same rate with the same chemical. What Does Ph Mean In Electrical Terms.
From microbenotes.com
pH Meter Principle, Parts, Procedure, Types, Uses, Examples What Does Ph Mean In Electrical Terms — as discussed, ph is the measurement of a specific ion (i.e., hydrogen). — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Some react violently, others moderately, and still others do not. Chemical solutions with ph levels below zero and above 14. The starting point is an electrochemical. What Does Ph Mean In Electrical Terms.
From www.positivemed.com
The Importance of pH! What Does Ph Mean In Electrical Terms As a result, the meaning of the name is justified as hydrogen power. Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. — ph is defined as the negative logarithm of the concentration of h+ ions. the basic ph scale. What Does Ph Mean In Electrical Terms.
From infinitylearn.com
pH Full Form Definition, Importance, Different Methods & Significance What Does Ph Mean In Electrical Terms We know that not all acids and bases react at the same rate with the same chemical compound. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. As a result, the meaning of the name is justified as hydrogen power. — the potential of hydrogen, or ph, is a measure of the concentration. What Does Ph Mean In Electrical Terms.
From psiberg.com
The pH Scale of Acid and Bases PSIBERG What Does Ph Mean In Electrical Terms The cell consists of an indicating electrode whose potential. As a result, the meaning of the name is justified as hydrogen power. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Some react violently, others moderately, and still others do not. — ph is defined as the negative. What Does Ph Mean In Electrical Terms.
From www.worksheetsplanet.com
What is PH Definition What Does Ph Mean In Electrical Terms Some react violently, others moderately, and still others do not. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. The starting point is an electrochemical cell. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). The term ph was. What Does Ph Mean In Electrical Terms.
From www.showme.com
Ph scale Science, Ph Scale ShowMe What Does Ph Mean In Electrical Terms — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. Chemical solutions with ph levels below zero and above 14. As a result, the meaning of the name is justified as hydrogen power. Some react. What Does Ph Mean In Electrical Terms.
From sciencenotes.org
The pH Scale of Common Chemicals What Does Ph Mean In Electrical Terms — ph is defined as the negative logarithm of the concentration of h+ ions. As a result, the meaning of the name is justified as hydrogen power. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. — as discussed, ph is the measurement of a specific ion. What Does Ph Mean In Electrical Terms.
From www.wiring-harness.net
What Does PH Mean In PH Terminal Wires Knowledge Ecocables What Does Ph Mean In Electrical Terms Some react violently, others moderately, and still others do not. We know that not all acids and bases react at the same rate with the same chemical compound. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. — ph is defined as the negative logarithm of the concentration of h+ ions. —. What Does Ph Mean In Electrical Terms.
From www.chemswot.com
Definition of pH scale in Chemistry What Does Ph Mean In Electrical Terms The starting point is an electrochemical cell. Chemical solutions with ph levels below zero and above 14. As a result, the meaning of the name is justified as hydrogen power. The cell consists of an indicating electrode whose potential. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. We. What Does Ph Mean In Electrical Terms.
From www.researchgate.net
Different pH values Conductivity Sensors Usually, conductivity is one What Does Ph Mean In Electrical Terms The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for. What Does Ph Mean In Electrical Terms.
From aqualife.ca
Discover the power of pH on your health Aqualife What Does Ph Mean In Electrical Terms — ph is defined as the negative logarithm of the concentration of h+ ions. Some react violently, others moderately, and still others do not. We know that not all acids and bases react at the same rate with the same chemical compound. As a result, the meaning of the name is justified as hydrogen power. The cell consists of. What Does Ph Mean In Electrical Terms.
From www.tffn.net
What Does pH Mean in Science? A Comprehensive Guide The Enlightened What Does Ph Mean In Electrical Terms Chemical solutions with ph levels below zero and above 14. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Some react violently, others moderately, and still others do not. As a result, the meaning of the name is justified as hydrogen power. The term ph was first described by. What Does Ph Mean In Electrical Terms.
From www.expii.com
What Is pH? — Definition & Overview Expii What Does Ph Mean In Electrical Terms Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. The cell consists of an indicating electrode whose potential. Some react violently, others moderately, and still others do not. Chemical solutions with ph levels below zero and above 14. — as discussed,. What Does Ph Mean In Electrical Terms.
From www.pmel.noaa.gov
The pH scale by numbers What Does Ph Mean In Electrical Terms Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). The term ph was first described by danish biochemist søren peter lauritz sørensen in. What Does Ph Mean In Electrical Terms.
From content.byui.edu
Acids, Bases, pH and Buffers What Does Ph Mean In Electrical Terms We know that not all acids and bases react at the same rate with the same chemical compound. The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Chemical solutions with ph levels below zero. What Does Ph Mean In Electrical Terms.
From preparatorychemistry.com
pH and Equilibrium What Does Ph Mean In Electrical Terms — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). Chemical solutions with ph levels below zero and above 14. The starting point is an electrochemical cell. — as discussed,. What Does Ph Mean In Electrical Terms.
From www.pmel.noaa.gov
The pH scale with some common examples What Does Ph Mean In Electrical Terms We know that not all acids and bases react at the same rate with the same chemical compound. The starting point is an electrochemical cell. Some react violently, others moderately, and still others do not. Chemical solutions with ph levels below zero and above 14. Ph is an abbreviation for power of hydrogen where p is short for the german. What Does Ph Mean In Electrical Terms.
From www.sciencenewsforstudents.org
Explainer What the pH scale tells us Science News for Students What Does Ph Mean In Electrical Terms As a result, the meaning of the name is justified as hydrogen power. We know that not all acids and bases react at the same rate with the same chemical compound. The starting point is an electrochemical cell. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. Chemical solutions. What Does Ph Mean In Electrical Terms.
From www.slideserve.com
PPT Chemistry Acids and Bases pH, pOH PowerPoint Presentation, free What Does Ph Mean In Electrical Terms Ph is an abbreviation for power of hydrogen where p is short for the german word for power, potenz and h is the element symbol for hydrogen. Some react violently, others moderately, and still others do not. — the potential of hydrogen, or ph, is a measure of the concentration of hydrogen ions in a solution. As a result,. What Does Ph Mean In Electrical Terms.
From www.youtube.com
ph scale and relation between ph and poh electrochemistry class 12 What Does Ph Mean In Electrical Terms The term ph was first described by danish biochemist søren peter lauritz sørensen in 1909. The starting point is an electrochemical cell. the basic ph scale extends from 0 (strong acid) to 7 (neutral, pure water) to 14 (strong caustic). The cell consists of an indicating electrode whose potential. — as discussed, ph is the measurement of a. What Does Ph Mean In Electrical Terms.