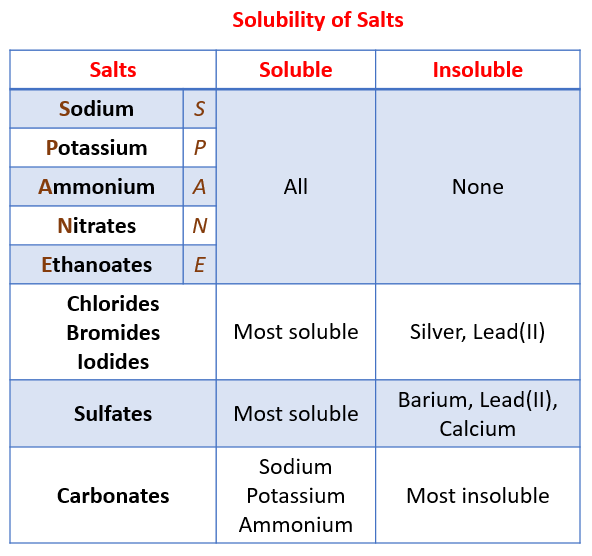
Which Salt Is Insoluble In Water . Titration must be used if the reactants are soluble. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. soluble salts can be made by reacting acids with soluble or insoluble reactants. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Most silver salts are insoluble. If i say one mole of sodium sulfate dissolves in water i am really meaning for. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. These rules are general and qualitative in nature. Agno3 and ag (c2h3o2) are common soluble salts of silver; Virtually all others are insoluble. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Most sulfate salts are soluble. 133 rows — what do we mean by solubility of a salt?
from www.onlinemathlearning.com
Most silver salts are insoluble. 133 rows — what do we mean by solubility of a salt? Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. These rules are general and qualitative in nature. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Titration must be used if the reactants are soluble. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Thus, agcl, pbbr2, and hg2cl2 are insoluble. If i say one mole of sodium sulfate dissolves in water i am really meaning for. Virtually all others are insoluble.
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets
Which Salt Is Insoluble In Water These rules are general and qualitative in nature. 133 rows — what do we mean by solubility of a salt? Most sulfate salts are soluble. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; soluble salts can be made by reacting acids with soluble or insoluble reactants. If i say one mole of sodium sulfate dissolves in water i am really meaning for. Virtually all others are insoluble. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Titration must be used if the reactants are soluble. Most silver salts are insoluble. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. These rules are general and qualitative in nature.
From www.youtube.com
Preparation of Salts /Soluble and insoluble salts/ Ionic Compounds Which Salt Is Insoluble In Water Titration must be used if the reactants are soluble. Virtually all others are insoluble. soluble salts can be made by reacting acids with soluble or insoluble reactants. These rules are general and qualitative in nature. Most sulfate salts are soluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; Most silver salts are insoluble. li 2co 3. Which Salt Is Insoluble In Water.
From www.slideshare.net
SPM Form 4 Chapter 8 Salt Which Salt Is Insoluble In Water a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. If i say one mole of sodium sulfate dissolves in water i am really meaning for. Virtually all others are insoluble. Titration must be used if the reactants are soluble. 133 rows. Which Salt Is Insoluble In Water.
From www.chemistrylearner.com
Solubility Rules Definition, Examples, and Table Which Salt Is Insoluble In Water Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Most sulfate salts are soluble. Titration must be used if the reactants are soluble. soluble salts can be made by reacting acids with soluble or insoluble reactants. a salt is soluble if it dissolves in water to give a solution with a concentration of at. Which Salt Is Insoluble In Water.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Which Salt Is Insoluble In Water soluble salts can be made by reacting acids with soluble or insoluble reactants. 133 rows — what do we mean by solubility of a salt? Virtually all others are insoluble. If i say one mole of sodium sulfate dissolves in water i am really meaning for. a salt is soluble if it dissolves in water to give. Which Salt Is Insoluble In Water.
From www.chegg.com
Solved Which salt is insoluble in water based on Table F in Which Salt Is Insoluble In Water Agno3 and ag (c2h3o2) are common soluble salts of silver; Titration must be used if the reactants are soluble. These rules are general and qualitative in nature. soluble salts can be made by reacting acids with soluble or insoluble reactants. Most sulfate salts are soluble. a salt is soluble if it dissolves in water to give a solution. Which Salt Is Insoluble In Water.
From riyakruwellis.blogspot.com
Is Salt Soluble in Water RiyakruwEllis Which Salt Is Insoluble In Water These rules are general and qualitative in nature. 133 rows — what do we mean by solubility of a salt? a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Virtually all others are insoluble. Thus, agcl, pbbr2, and hg2cl2 are insoluble.. Which Salt Is Insoluble In Water.
From www.slideshare.net
What is Salt? Which Salt Is Insoluble In Water Virtually all others are insoluble. If i say one mole of sodium sulfate dissolves in water i am really meaning for. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; Most silver salts are insoluble. Most sulfate salts are soluble. a salt is soluble if it dissolves in water to give. Which Salt Is Insoluble In Water.
From www.slideserve.com
PPT Chemical Reactions Chapter 7 PowerPoint Presentation, free Which Salt Is Insoluble In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for. These rules are general and qualitative in nature. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Most sulfate salts are soluble. a salt is soluble if it dissolves in water to. Which Salt Is Insoluble In Water.
From www.slideshare.net
8.1 (c) insoluble salts Which Salt Is Insoluble In Water Agno3 and ag (c2h3o2) are common soluble salts of silver; Thus, agcl, pbbr2, and hg2cl2 are insoluble. 133 rows — what do we mean by solubility of a salt? a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. li 2co. Which Salt Is Insoluble In Water.
From www.slideserve.com
PPT Salts PowerPoint Presentation ID1487768 Which Salt Is Insoluble In Water Most silver salts are insoluble. Virtually all others are insoluble. These rules are general and qualitative in nature. Titration must be used if the reactants are soluble. soluble salts can be made by reacting acids with soluble or insoluble reactants. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; If i. Which Salt Is Insoluble In Water.
From www.onlinemathlearning.com
Acid, Bases, Salts IGCSE Chemistry (solutions, examples, worksheets Which Salt Is Insoluble In Water soluble salts can be made by reacting acids with soluble or insoluble reactants. Virtually all others are insoluble. If i say one mole of sodium sulfate dissolves in water i am really meaning for. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Agno3 and ag (c2h3o2) are common soluble salts of silver; Most sulfate. Which Salt Is Insoluble In Water.
From www.slideserve.com
PPT Solutions PowerPoint Presentation ID6888319 Which Salt Is Insoluble In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for. Agno3 and ag (c2h3o2) are common soluble salts of silver; li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Thus, agcl, pbbr2, and. Which Salt Is Insoluble In Water.
From www.nagwa.com
Question Video Determining the Group of Elements Which Form Soluble Which Salt Is Insoluble In Water li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Virtually all others are insoluble. Most silver salts are insoluble. soluble salts can be made by reacting acids with soluble or insoluble reactants. Important exceptions to this rule are. Which Salt Is Insoluble In Water.
From www.pinterest.at
Insoluble salts. Chemistry, Aqa, Fate Which Salt Is Insoluble In Water Agno3 and ag (c2h3o2) are common soluble salts of silver; soluble salts can be made by reacting acids with soluble or insoluble reactants. 133 rows — what do we mean by solubility of a salt? Most sulfate salts are soluble. Titration must be used if the reactants are soluble. Virtually all others are insoluble. li 2co 3. Which Salt Is Insoluble In Water.
From www.labkafe.com
Solubility of Salts ‒ Why Common Salts are So Soluble in Water Labkafe Which Salt Is Insoluble In Water soluble salts can be made by reacting acids with soluble or insoluble reactants. Thus, agcl, pbbr2, and hg2cl2 are insoluble. 133 rows — what do we mean by solubility of a salt? Agno3 and ag (c2h3o2) are common soluble salts of silver; Most sulfate salts are soluble. a salt is soluble if it dissolves in water to. Which Salt Is Insoluble In Water.
From quinntewallace.blogspot.com
Soluble Salts List QuinnteWallace Which Salt Is Insoluble In Water Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Titration must be used if the reactants are soluble. soluble salts can be made by reacting acids with soluble or insoluble reactants. Most sulfate salts are soluble. Most silver salts are insoluble. li 2co 3 (s) ⇌ 2li. Which Salt Is Insoluble In Water.
From www.numerade.com
SOLVED Which of the following salts is insoluble in water? Use the Which Salt Is Insoluble In Water These rules are general and qualitative in nature. 133 rows — what do we mean by solubility of a salt? Virtually all others are insoluble. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Most sulfate salts are soluble. Most silver salts are insoluble. a salt is soluble if it dissolves in water to give a solution with a concentration. Which Salt Is Insoluble In Water.
From www.sliderbase.com
Solutions and solubility Presentation Chemistry Which Salt Is Insoluble In Water Virtually all others are insoluble. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Agno3 and ag (c2h3o2) are common soluble salts of silver; If i. Which Salt Is Insoluble In Water.
From www.oakabooks.co.uk
KS4/GCSE Making an Insoluble Salt Revision Resource For Dyslexics Which Salt Is Insoluble In Water Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. soluble salts can be made by reacting acids with soluble or insoluble reactants. Most silver salts are insoluble. These rules are general and qualitative in nature. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; 133 rows. Which Salt Is Insoluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID5581895 Which Salt Is Insoluble In Water Most sulfate salts are soluble. Virtually all others are insoluble. Titration must be used if the reactants are soluble. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Most silver salts are insoluble. a salt is soluble if it dissolves in water to give a solution with a. Which Salt Is Insoluble In Water.
From www.vectorstock.com
How does sodium chloride nacl dissolve in water Vector Image Which Salt Is Insoluble In Water li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Most silver salts are insoluble. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. 133 rows — what. Which Salt Is Insoluble In Water.
From manualfixporterly55.z22.web.core.windows.net
Equation For Salt Dissolving In Water Which Salt Is Insoluble In Water Most sulfate salts are soluble. These rules are general and qualitative in nature. Titration must be used if the reactants are soluble. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1. Which Salt Is Insoluble In Water.
From www.youtube.com
Solubility of salts and preparation of soluble salts YouTube Which Salt Is Insoluble In Water Most sulfate salts are soluble. Titration must be used if the reactants are soluble. These rules are general and qualitative in nature. Virtually all others are insoluble. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Important exceptions to this rule are. Which Salt Is Insoluble In Water.
From studylibplimsoll.z21.web.core.windows.net
What Is Insoluble Salt Which Salt Is Insoluble In Water Thus, agcl, pbbr2, and hg2cl2 are insoluble. Most silver salts are insoluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; Most sulfate salts are soluble. soluble salts can be made by reacting acids with soluble or insoluble reactants. Virtually all others are insoluble. a salt is soluble if it dissolves in water to give a solution. Which Salt Is Insoluble In Water.
From studymind.co.uk
ᐉ Solubility Rules Insoluble & Soluble Salts Making Which Salt Is Insoluble In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Agno3 and ag (c2h3o2) are common soluble salts of silver; Thus, agcl, pbbr2, and. Which Salt Is Insoluble In Water.
From mungfali.com
Salt Solubility Chart Which Salt Is Insoluble In Water a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Thus, agcl, pbbr2,. Which Salt Is Insoluble In Water.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Which Salt Is Insoluble In Water Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Thus, agcl, pbbr2, and hg2cl2 are insoluble. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Most sulfate salts are soluble. li 2co 3 (s) ⇌ 2li +. Which Salt Is Insoluble In Water.
From userdatarheumatics.z21.web.core.windows.net
Diagram Of Salt Dissolving In Water Which Salt Is Insoluble In Water Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. Most silver salts are insoluble. These rules are general and qualitative in nature. Most sulfate salts are. Which Salt Is Insoluble In Water.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Which Salt Is Insoluble In Water li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. These rules are general and qualitative in nature. soluble salts can be made by reacting acids with soluble or insoluble reactants. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Titration. Which Salt Is Insoluble In Water.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Which Salt Is Insoluble In Water Titration must be used if the reactants are soluble. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. 133 rows — what do we mean by solubility of a salt? a salt is soluble if it dissolves. Which Salt Is Insoluble In Water.
From www.youtube.com
GCSE CHEMISTRY ACIDS AND BASES LESSON 19 salts solubility YouTube Which Salt Is Insoluble In Water If i say one mole of sodium sulfate dissolves in water i am really meaning for. Most silver salts are insoluble. Most sulfate salts are soluble. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. 133 rows — what do we mean by solubility of a salt? soluble salts can be made by reacting. Which Salt Is Insoluble In Water.
From www.alamy.com
Solution science experiment. Solubility of salt and sand in water Which Salt Is Insoluble In Water Most sulfate salts are soluble. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. These rules are general and qualitative in nature. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+.. Which Salt Is Insoluble In Water.
From quizunderslung.z4.web.core.windows.net
Insoluble Salts List Which Salt Is Insoluble In Water These rules are general and qualitative in nature. Virtually all others are insoluble. a salt is soluble if it dissolves in water to give a solution with a concentration of at least 0.1 moles per liter at room temperature. Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. soluble salts can be made by. Which Salt Is Insoluble In Water.
From www.differencebetween.com
Difference Between Soluble and Insoluble Salts Compare the Difference Which Salt Is Insoluble In Water Most sulfate salts are soluble. These rules are general and qualitative in nature. li 2co 3 (s) ⇌ 2li + (aq) + co2 − 3 (aq) [li +]2[co − 3] the solubility product, ksp, is a special type of equilibrium constant given. If i say one mole of sodium sulfate dissolves in water i am really meaning for. Agno3. Which Salt Is Insoluble In Water.
From www.slideserve.com
PPT Soluble Salts in Water PowerPoint Presentation, free download Which Salt Is Insoluble In Water 133 rows — what do we mean by solubility of a salt? Important exceptions to this rule are halide salts of ag+, pb2+, and (hg2)2+. Thus, agcl, pbbr2, and hg2cl2 are insoluble. Agno3 and ag (c2h3o2) are common soluble salts of silver; soluble salts can be made by reacting acids with soluble or insoluble reactants. Titration must be. Which Salt Is Insoluble In Water.