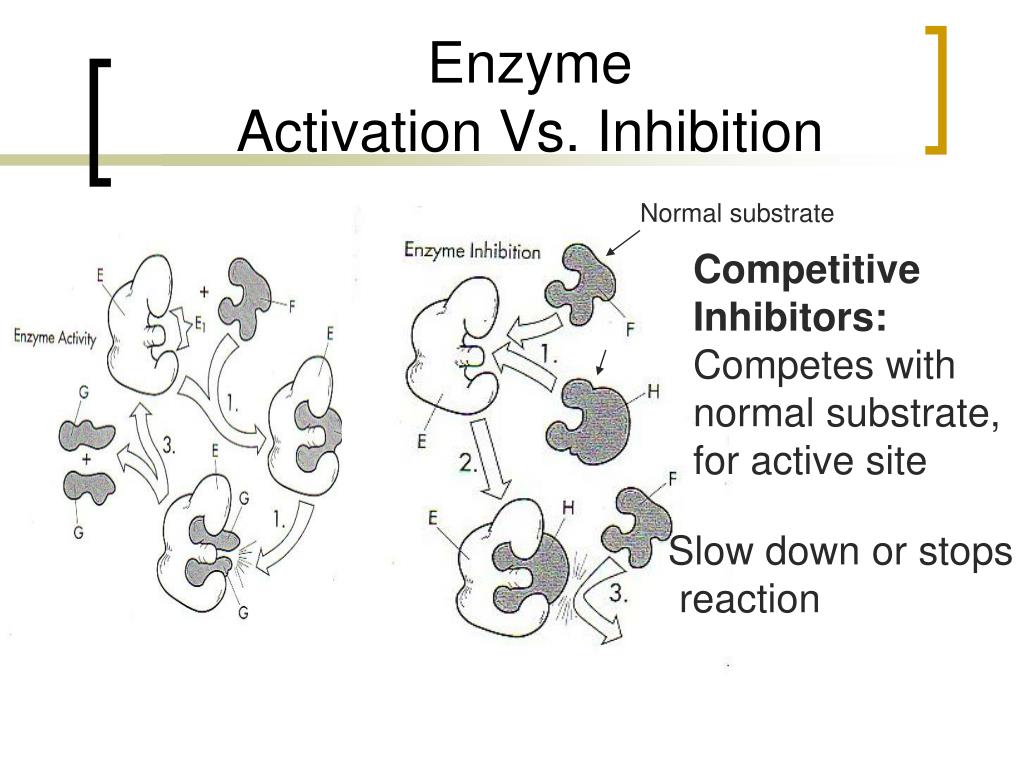
Catalysis Enzyme Inhibition . with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. When an inhibitor interacts with an enzyme it decreases the enzyme’s. the actions of many drugs involve enzyme inhibition. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. elucidating mechanisms for the inhibition of enzyme catalysis. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. This is exemplified by the inhibitors of monoamine oxidases. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and.
from www.slideserve.com
This is exemplified by the inhibitors of monoamine oxidases. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. the actions of many drugs involve enzyme inhibition. elucidating mechanisms for the inhibition of enzyme catalysis. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. When an inhibitor interacts with an enzyme it decreases the enzyme’s. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an.
PPT Enzymes, Nature’s Catalyst PowerPoint Presentation, free download
Catalysis Enzyme Inhibition with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. This is exemplified by the inhibitors of monoamine oxidases. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. the actions of many drugs involve enzyme inhibition. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. When an inhibitor interacts with an enzyme it decreases the enzyme’s. elucidating mechanisms for the inhibition of enzyme catalysis. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an.
From www.slideserve.com
PPT Enzymes, Regulation, And Inhibition By Nic Oliver And Jamie Catalysis Enzyme Inhibition the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. elucidating mechanisms for the. Catalysis Enzyme Inhibition.
From www.dreamstime.com
Enzyme Function. Macromolecular Biological Catalysts. Stock Catalysis Enzyme Inhibition In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. the actions of many. Catalysis Enzyme Inhibition.
From www.dreamstime.com
Catalyst Competitive Inhibition Active Site Stock Vector Illustration Catalysis Enzyme Inhibition reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. elucidating mechanisms for the inhibition of enzyme catalysis. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. This is exemplified by the inhibitors of. Catalysis Enzyme Inhibition.
From www.scribd.com
Enzyme Catalysis PDF Enzyme Inhibitor Enzyme Catalysis Enzyme Inhibition with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. When an inhibitor interacts with an enzyme it decreases the enzyme’s. This is exemplified by the inhibitors of monoamine oxidases. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as. Catalysis Enzyme Inhibition.
From www.sciencemotive.com
Catalyst and Mechanism of Enzyme Catalysis ScienceMotive Catalysis Enzyme Inhibition This is exemplified by the inhibitors of monoamine oxidases. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. reversible competitive inhibition occurs when substrate (s) and inhibitor. Catalysis Enzyme Inhibition.
From biotechnologymcq.com
Enzyme V(II) Catalytic Mechanisms II Metal Ion catalysis; Catalysis by Catalysis Enzyme Inhibition This is exemplified by the inhibitors of monoamine oxidases. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. protease inhibitors can act in several ways, including as a suicide inhibitor,. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT Lecture 12 Enzyme Catalysis PowerPoint Presentation, free Catalysis Enzyme Inhibition with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. This is exemplified by the inhibitors of monoamine oxidases. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by. Catalysis Enzyme Inhibition.
From www.youtube.com
ENZYME CATALYSIS Competitive and non competitive inhibition Catalysis Enzyme Inhibition This is exemplified by the inhibitors of monoamine oxidases. elucidating mechanisms for the inhibition of enzyme catalysis. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on. Catalysis Enzyme Inhibition.
From fity.club
Enzyme The Catalyst Catalysis Enzyme Inhibition elucidating mechanisms for the inhibition of enzyme catalysis. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. the catalytic efficiency (proficiency, specificity) of. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalysis Enzyme Inhibition with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. reversible competitive inhibition occurs. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT ENZYME CATALYSIS PowerPoint Presentation, free download ID4176592 Catalysis Enzyme Inhibition reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. This is exemplified by the inhibitors of monoamine oxidases. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. with noncompetitive inhibition the substrate and the inhibitor bind to different. Catalysis Enzyme Inhibition.
From teachmephysiology.com
Enzyme Inhibition Types of Inhibition TeachMePhysiology Catalysis Enzyme Inhibition elucidating mechanisms for the inhibition of enzyme catalysis. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. the actions of many drugs involve enzyme inhibition. This is exemplified by the. Catalysis Enzyme Inhibition.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysis Enzyme Inhibition In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km.. Catalysis Enzyme Inhibition.
From www.slideshare.net
Basics of Enzyme Catalysis PPT Catalysis Enzyme Inhibition protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. elucidating mechanisms for the inhibition of enzyme catalysis. This is exemplified by the inhibitors of monoamine oxidases. . Catalysis Enzyme Inhibition.
From infinitabiotech.com
Characteristics Of Enzyme Catalysis Infinita Biotech Catalysis Enzyme Inhibition the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as. Catalysis Enzyme Inhibition.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalysis Enzyme Inhibition elucidating mechanisms for the inhibition of enzyme catalysis. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. This is exemplified by the inhibitors of monoamine oxidases. the actions of many drugs involve enzyme inhibition. protease inhibitors can act in several ways, including as a suicide inhibitor, a. Catalysis Enzyme Inhibition.
From dxopopljn.blob.core.windows.net
Enzyme Catalyzed Reactions at Richard Fountain blog Catalysis Enzyme Inhibition elucidating mechanisms for the inhibition of enzyme catalysis. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the. Catalysis Enzyme Inhibition.
From www.sliderbase.com
Enzymes. A Cell's Catalysts Presentation Biology Catalysis Enzyme Inhibition the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. When an inhibitor interacts with an enzyme it decreases the enzyme’s. elucidating mechanisms for the inhibition of enzyme catalysis. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. This is. Catalysis Enzyme Inhibition.
From slidetodoc.com
LECTURE 2 ENZYME GENERAL PRINCIPLES OF CATALYSIS Catalysis Enzyme Inhibition with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. When an inhibitor interacts with an enzyme it decreases the enzyme’s. the actions of many drugs involve enzyme inhibition. This is exemplified by the inhibitors of monoamine oxidases. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst). Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT Enzymes, Nature’s Catalyst PowerPoint Presentation, free download Catalysis Enzyme Inhibition elucidating mechanisms for the inhibition of enzyme catalysis. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. with noncompetitive inhibition the substrate and the inhibitor bind. Catalysis Enzyme Inhibition.
From courses.lumenlearning.com
Enzymes OpenStax Biology 2e Catalysis Enzyme Inhibition This is exemplified by the inhibitors of monoamine oxidases. When an inhibitor interacts with an enzyme it decreases the enzyme’s. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. protease inhibitors can. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT Glycolysis Regulation PowerPoint Presentation, free download Catalysis Enzyme Inhibition with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. the actions of many drugs involve enzyme inhibition. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. This is exemplified by the inhibitors of monoamine oxidases. protease inhibitors can act in. Catalysis Enzyme Inhibition.
From favpng.com
Enzyme Inhibitor Dihydrofolate Reductase Catalysis Active Site, PNG Catalysis Enzyme Inhibition the actions of many drugs involve enzyme inhibition. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. elucidating mechanisms for the inhibition of enzyme catalysis. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating. Catalysis Enzyme Inhibition.
From zymvol.com
All you need to know about enzymes Zymvol Catalysis Enzyme Inhibition the actions of many drugs involve enzyme inhibition. When an inhibitor interacts with an enzyme it decreases the enzyme’s. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. . Catalysis Enzyme Inhibition.
From www.researchgate.net
Proposed mechanisms for the catalystenzyme coupled system in catalytic Catalysis Enzyme Inhibition In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. elucidating mechanisms for the inhibition of enzyme catalysis. When an inhibitor interacts with an enzyme it decreases the enzyme’s. the actions of many drugs involve enzyme inhibition. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to. Catalysis Enzyme Inhibition.
From www.biologyexams4u.com
Reversible Enzyme Inhibition Competitive, Non Competitive and Catalysis Enzyme Inhibition the actions of many drugs involve enzyme inhibition. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. elucidating mechanisms for the inhibition of enzyme catalysis. In the first step,. Catalysis Enzyme Inhibition.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Catalysis Enzyme Inhibition reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. This is exemplified by the inhibitors of monoamine oxidases. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. with noncompetitive inhibition the substrate and. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT Enzyme Catalysis (26.4) PowerPoint Presentation, free download Catalysis Enzyme Inhibition This is exemplified by the inhibitors of monoamine oxidases. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. reversible competitive inhibition occurs when substrate (s). Catalysis Enzyme Inhibition.
From www.studocu.com
Chemistry 3 Thermodynamics and Mechanism of Enzyme Catalysis, Enzyme Catalysis Enzyme Inhibition the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. the actions of many drugs involve enzyme inhibition. elucidating mechanisms for the inhibition of enzyme catalysis. When an inhibitor interacts with an. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT Enzyme Catalysis PowerPoint Presentation, free download ID6835497 Catalysis Enzyme Inhibition reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. elucidating mechanisms for. Catalysis Enzyme Inhibition.
From theoryanalysis.netlify.app
What is involved in enzyme catalysis Catalysis Enzyme Inhibition In the first step, an enzyme molecule (e) and the substrate molecule or molecules (s) collide and. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. elucidating mechanisms for the inhibition of enzyme catalysis. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT ENZYMES INHIBITION, REGULATION PowerPoint Presentation Catalysis Enzyme Inhibition reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. This is exemplified by the inhibitors of monoamine oxidases. elucidating mechanisms for the inhibition of enzyme catalysis. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. When an inhibitor. Catalysis Enzyme Inhibition.
From www.expii.com
Enzyme Inhibition — Overview & Types Expii Catalysis Enzyme Inhibition reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. the actions of many drugs involve enzyme inhibition. with noncompetitive inhibition the substrate and. Catalysis Enzyme Inhibition.
From www.slideserve.com
PPT ENZYME CATALYSIS PowerPoint Presentation, free download ID7121999 Catalysis Enzyme Inhibition When an inhibitor interacts with an enzyme it decreases the enzyme’s. with noncompetitive inhibition the substrate and the inhibitor bind to different active sites on the enzyme, forming an. the catalytic efficiency (proficiency, specificity) of an enzyme (or any catalyst) is given by the kinetic parameter kcat/km. the actions of many drugs involve enzyme inhibition. elucidating. Catalysis Enzyme Inhibition.
From www.dreamstime.com
Enzyme Catalyst Active Site for Binding Reactants Stock Vector Catalysis Enzyme Inhibition When an inhibitor interacts with an enzyme it decreases the enzyme’s. reversible competitive inhibition occurs when substrate (s) and inhibitor (i) both bind to the same site on the enzyme. protease inhibitors can act in several ways, including as a suicide inhibitor, a transition state inhibitor, a denaturant, and as a chelating agent. In the first step, an. Catalysis Enzyme Inhibition.