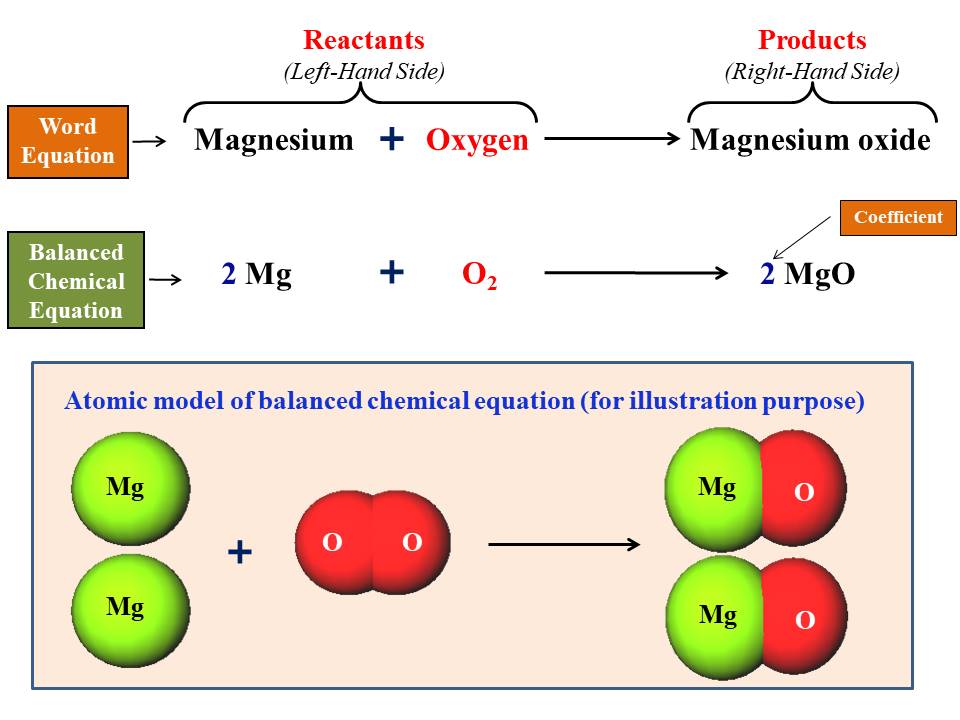
What Is Chemical Balance . Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. We can relate a balanced chemical. A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. The coefficients in a balanced equation must be. Explain the role of the law of conservation of matter in a. Balanced chemical equations have the same number and type of each atom on both sides of the equation. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Balance any equation or reaction using this chemical equation balancer! A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Find out what type of reaction occured.
from www.scibond.com
We can relate a balanced chemical. Find out what type of reaction occured. Balanced chemical equations have the same number and type of each atom on both sides of the equation. Balance any equation or reaction using this chemical equation balancer! Balance a chemical equation when given the unbalanced equation. The coefficients in a balanced equation must be. Explain the role of the law of conservation of matter in a. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Explain the roles of subscripts and coefficients in chemical equations. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and.
How to Balance a Chemical Equations? SciBond
What Is Chemical Balance A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. The coefficients in a balanced equation must be. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Balanced chemical equations have the same number and type of each atom on both sides of the equation. Find out what type of reaction occured. We can relate a balanced chemical. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Balance a chemical equation when given the unbalanced equation. Explain the roles of subscripts and coefficients in chemical equations. A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. Explain the role of the law of conservation of matter in a. Balance any equation or reaction using this chemical equation balancer!
From www.chemicals.co.uk
Simple Steps to Balance Chemical Equations The Chemistry Blog What Is Chemical Balance A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. We can relate a balanced chemical. Balanced chemical equations have the same number and type of each atom on both sides of the equation. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers. What Is Chemical Balance.
From study.com
Balancing Chemical Equations Definition, Process & Examples Lesson What Is Chemical Balance A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Find out what type of reaction occured. Balanced chemical equations have the same number and type of each atom on both sides of the equation. Explain. What Is Chemical Balance.
From www.youtube.com
How to Balance Chemical Reactions (in 2 minutes)! YouTube What Is Chemical Balance Explain the role of the law of conservation of matter in a. A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. Find out what type of reaction occured. Balanced chemical equations have the same number and type of each atom on both sides of the equation. Explain the roles of. What Is Chemical Balance.
From chemnotcheem.com
How to balance chemical equations O Level Chemistry Notes What Is Chemical Balance A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. Explain the roles of subscripts and coefficients in chemical equations. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! Explain the role of the law of conservation of matter in a. Balance a. What Is Chemical Balance.
From science.wonderhowto.com
How to Properly balance chemical equations « Science Experiments What Is Chemical Balance Balance a chemical equation when given the unbalanced equation. Explain the role of the law of conservation of matter in a. We can relate a balanced chemical. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. A balanced chemical equation shows. What Is Chemical Balance.
From www.studypug.com
Master Balancing Chemical Equations StepbyStep Guide StudyPug What Is Chemical Balance A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Balanced chemical equations have the same number and type of each atom on both sides of the equation. Explain the role of the law of conservation. What Is Chemical Balance.
From www.wikihow.com
How to Balance Chemical Equations 10 Steps (with Pictures) What Is Chemical Balance We can relate a balanced chemical. The coefficients in a balanced equation must be. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Balanced chemical equations have the same number and type of each atom on both sides of the equation.. What Is Chemical Balance.
From geteducationbee.com
How to Balance Chemical Equations? Best Examples Get Education Bee What Is Chemical Balance We can relate a balanced chemical. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Balance a chemical equation when given the unbalanced equation. Find out what type of reaction occured. Balanced chemical equations have. What Is Chemical Balance.
From www.youtube.com
How To Balance Chemical Equations YouTube What Is Chemical Balance A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of. What Is Chemical Balance.
From www.youtube.com
How to Balance Chemical Equations YouTube What Is Chemical Balance A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. We can relate a balanced chemical. Find out what type of reaction occured. Balance a chemical equation when given the unbalanced equation. A balanced chemical equation shows the same number of each. What Is Chemical Balance.
From quizlet.com
Chemistry balance chemical equation Diagram Quizlet What Is Chemical Balance Balanced chemical equations have the same number and type of each atom on both sides of the equation. The coefficients in a balanced equation must be. Find out what type of reaction occured. Balance a chemical equation when given the unbalanced equation. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of. What Is Chemical Balance.
From www.biolineindia.com
CHEMICAL BALANCE Bioline India What Is Chemical Balance Find out what type of reaction occured. We can relate a balanced chemical. Balance any equation or reaction using this chemical equation balancer! Explain the role of the law of conservation of matter in a. Balance a chemical equation when given the unbalanced equation. The coefficients in a balanced equation must be. Explain the roles of subscripts and coefficients in. What Is Chemical Balance.
From halomedicals.com
Chemical Weighing Balance HALOMEDICALS SYSTEMS LIMITED What Is Chemical Balance Explain the roles of subscripts and coefficients in chemical equations. Balance any equation or reaction using this chemical equation balancer! A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Find out what type of reaction. What Is Chemical Balance.
From www.tessshebaylo.com
How To Balance Equations In Chemistry Bbc Bitesize Tessshebaylo What Is Chemical Balance We can relate a balanced chemical. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Explain the role of the law of conservation of matter in a. A balanced equation is a chemical equation in. What Is Chemical Balance.
From www.onlinemathlearning.com
How to balance chemical equations (solutions, examples, videos) What Is Chemical Balance The coefficients in a balanced equation must be. We can relate a balanced chemical. Balance any equation or reaction using this chemical equation balancer! A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Explain the. What Is Chemical Balance.
From www.slideserve.com
PPT How to Balance Chemical Equations PowerPoint Presentation, free What Is Chemical Balance Balance any equation or reaction using this chemical equation balancer! A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. We can relate a balanced chemical. Balanced chemical equations have the same number and type of. What Is Chemical Balance.
From vhmsscience8.weebly.com
Balancing Chemical Equations VISTA HEIGHTS 8TH GRADE SCIENCE What Is Chemical Balance The coefficients in a balanced equation must be. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Balance a chemical equation when given the unbalanced equation. Find out what type of reaction occured. We can. What Is Chemical Balance.
From printableveselig9n.z13.web.core.windows.net
50 Examples Of Balanced Chemical Equations What Is Chemical Balance Find out what type of reaction occured. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Explain the roles of subscripts and coefficients in chemical equations. Explain the role of the law of conservation of matter in a. A balanced equation. What Is Chemical Balance.
From www.microteknik.com
ChemicalBalanceWithWeight What Is Chemical Balance We can relate a balanced chemical. A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. Explain the role of the law of conservation of matter in a. Balance a chemical equation when given the unbalanced equation. Balance any equation or reaction using this chemical equation balancer! The coefficients in a. What Is Chemical Balance.
From www.youtube.com
C + H2 = CH4 Balancing Equations YouTube What Is Chemical Balance A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. We can relate a balanced chemical. Explain the role of the law of conservation of matter in a. Balance a chemical equation when given the unbalanced equation. A balanced equation is an. What Is Chemical Balance.
From vhmsscience8.weebly.com
Balancing Chemical Equations VISTA HEIGHTS 8TH GRADE SCIENCE What Is Chemical Balance A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Balanced chemical equations have the same number and type of each atom on. What Is Chemical Balance.
From www.scibond.com
How to Balance a Chemical Equations? SciBond What Is Chemical Balance Balanced chemical equations have the same number and type of each atom on both sides of the equation. Explain the role of the law of conservation of matter in a. A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. A balanced equation is a chemical equation in which mass is. What Is Chemical Balance.
From www.typecalendar.com
Printable Templates For Balancing Chemical Equations Master Chemistry What Is Chemical Balance We can relate a balanced chemical. The coefficients in a balanced equation must be. Balance a chemical equation when given the unbalanced equation. A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Balance any equation or reaction using this chemical equation. What Is Chemical Balance.
From www.scibond.com
How to Balance a Chemical Equations? SciBond What Is Chemical Balance Balance a chemical equation when given the unbalanced equation. Balance any equation or reaction using this chemical equation balancer! A balanced chemical equation shows the same number of each type of atom on both sides of the arrow. Find out what type of reaction occured. Explain the role of the law of conservation of matter in a. The coefficients in. What Is Chemical Balance.
From www.wikihow.com
How to Balance Chemical Equations 11 Steps (with Pictures) What Is Chemical Balance Balanced chemical equations have the same number and type of each atom on both sides of the equation. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Find out what type of reaction occured. A. What Is Chemical Balance.
From www.slideserve.com
PPT How to Balance Chemical Equations PowerPoint Presentation, free What Is Chemical Balance Balanced chemical equations have the same number and type of each atom on both sides of the equation. The coefficients in a balanced equation must be. Find out what type of reaction occured. We can relate a balanced chemical. Balance a chemical equation when given the unbalanced equation. Balance any equation or reaction using this chemical equation balancer! A balanced. What Is Chemical Balance.
From onlinesciencenotes.com
Chemical reactions, balanced and unbalanced chemical equations Online What Is Chemical Balance Balance any equation or reaction using this chemical equation balancer! A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. We can relate a balanced chemical. Explain the roles of subscripts and coefficients in chemical equations.. What Is Chemical Balance.
From www.youtube.com
Balancing Chemical Equations 2 YouTube What Is Chemical Balance Balance a chemical equation when given the unbalanced equation. Balanced chemical equations have the same number and type of each atom on both sides of the equation. Find out what type of reaction occured. Balance any equation or reaction using this chemical equation balancer! We can relate a balanced chemical. The coefficients in a balanced equation must be. A balanced. What Is Chemical Balance.
From www.youtube.com
Balancing Chemical Equations YouTube What Is Chemical Balance We can relate a balanced chemical. Balance any equation or reaction using this chemical equation balancer! Balanced chemical equations have the same number and type of each atom on both sides of the equation. The coefficients in a balanced equation must be. Find out what type of reaction occured. A balanced chemical equation shows the same number of each type. What Is Chemical Balance.
From www.wikihow.com
How to Balance Chemical Equations 11 Steps (with Pictures) What Is Chemical Balance A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. Explain the roles of subscripts and coefficients in chemical equations. The coefficients in a balanced equation must be. We can relate a balanced chemical. Find out what type of reaction occured. A. What Is Chemical Balance.
From www.windward.solutions
Balancing chemical equations kahoot What Is Chemical Balance Explain the roles of subscripts and coefficients in chemical equations. Find out what type of reaction occured. Explain the role of the law of conservation of matter in a. We can relate a balanced chemical. The coefficients in a balanced equation must be. A balanced equation is an equation for a chemical reaction in which the number of atoms for. What Is Chemical Balance.
From chemsimplified.com
Balancing Chemical Equations ChemSimplified What Is Chemical Balance A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. Balanced chemical equations have the same number and type of each atom on both sides of the equation. The coefficients in a balanced equation must be.. What Is Chemical Balance.
From www.youtube.com
How to Balance Chemical Equations YouTube What Is Chemical Balance A balanced equation is a chemical equation in which mass is conserved and there are equal numbers of atoms of each element on both sides of the equation. We can relate a balanced chemical. The coefficients in a balanced equation must be. Balanced chemical equations have the same number and type of each atom on both sides of the equation.. What Is Chemical Balance.
From www.expii.com
Balancing Chemical Equations — Overview & Examples Expii What Is Chemical Balance Balanced chemical equations have the same number and type of each atom on both sides of the equation. We can relate a balanced chemical. Explain the roles of subscripts and coefficients in chemical equations. A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge. What Is Chemical Balance.
From www.online-sciences.com
Chemical Equilibrium, Chemical reactions types, complete reactions and What Is Chemical Balance A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and. The coefficients in a balanced equation must be. Find out what type of reaction occured. Balanced chemical equations have the same number and type of each. What Is Chemical Balance.