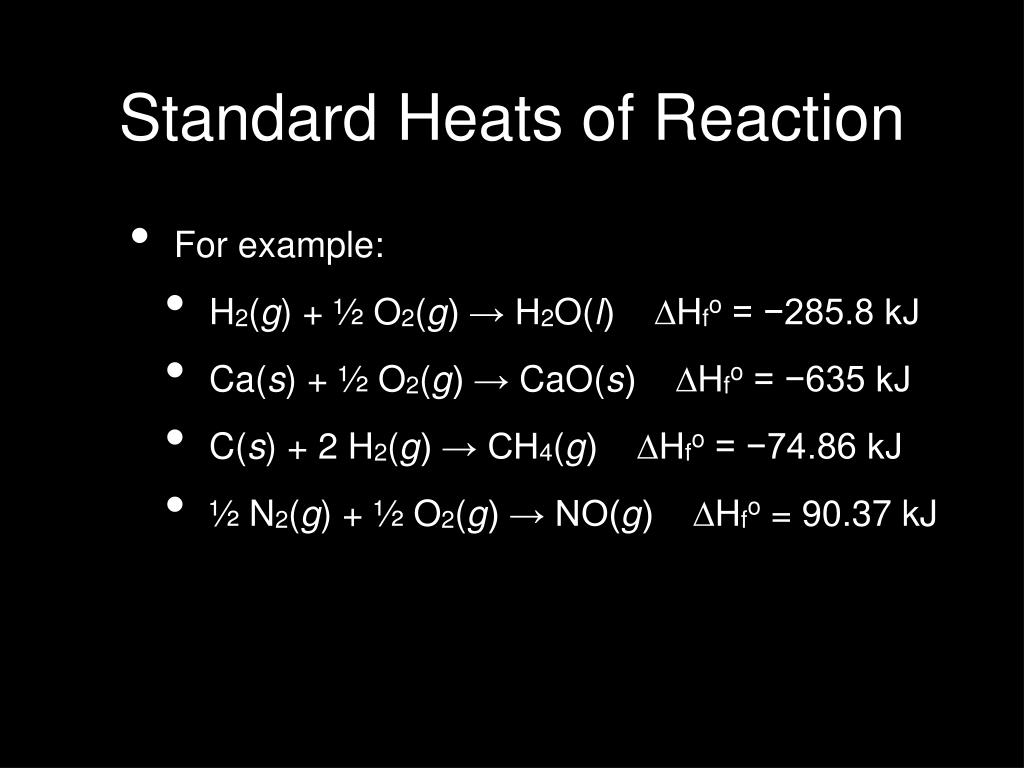
Standard Heat Of Formation Example . For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. By the way, standard enthalpies of various substances. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and.
from www.slideserve.com
The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By the way, standard enthalpies of various substances. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition.
PPT Calculating Heats of Reaction PowerPoint Presentation, free
Standard Heat Of Formation Example For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. By the way, standard enthalpies of various substances. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Standard Heat Of Formation Example For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of any element in its standard state is zero by definition. By the way, standard enthalpies of various substances. The standard enthalpy of formation of any. Standard Heat Of Formation Example.
From www.youtube.com
Writing Equations for Standard Enthalpy of Formation Examples YouTube Standard Heat Of Formation Example The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Heat Of Formation Example.
From slideplayer.com
Chapter 17 “Thermochemistry” ppt download Standard Heat Of Formation Example By the way, standard enthalpies of various substances. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of. Standard Heat Of Formation Example.
From www.slideshare.net
Standard enthalpy of formation Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. By the way, standard enthalpies of various substances. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is. Standard Heat Of Formation Example.
From www.slideserve.com
PPT CHAPTER 4 PowerPoint Presentation ID3180471 Standard Heat Of Formation Example For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Example By the way, standard enthalpies of various substances. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although. Standard Heat Of Formation Example.
From studyschoolburman.z21.web.core.windows.net
Formula Of Heat Of Formation Standard Heat Of Formation Example For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. By the way, standard enthalpies of various substances. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, although oxygen can exist as. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Heat Of Formation Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation of. Standard Heat Of Formation Example.
From www.slideserve.com
PPT STANDARD HEAT OF FORMATION ΔH 0 f or ΔH θ f PowerPoint Standard Heat Of Formation Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. By the way, standard enthalpies of various substances. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. For example, here is a link to obtain the. Standard Heat Of Formation Example.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Standard Heat Of Formation Example Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. By the way, standard enthalpies of various substances. The standard enthalpy of formation of a pure. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Ch. 15 Energy and Chemical Change PowerPoint Presentation, free Standard Heat Of Formation Example By the way, standard enthalpies of various substances. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. Standard enthalpy of formation (or heat of. Standard Heat Of Formation Example.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. By the way, standard enthalpies of various substances. The standard enthalpy of formation of a. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Calculating Heats of Reaction PowerPoint Presentation, free Standard Heat Of Formation Example For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation, also known as the heat of formation, is. Standard Heat Of Formation Example.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Heat Of Formation Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. The standard enthalpy of formation of. Standard Heat Of Formation Example.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Heat Of Formation Example The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID314871 Standard Heat Of Formation Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, here is a link. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Energy Transformations PowerPoint Presentation, free download Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and.. Standard Heat Of Formation Example.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. By the way, standard enthalpies of various substances. 193 rows in chemistry. Standard Heat Of Formation Example.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation of any element in its standard state is zero by. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Chemistry 17.4 PowerPoint Presentation, free download ID2772524 Standard Heat Of Formation Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation,. Standard Heat Of Formation Example.
From slideplayer.com
Chapter 10 “Thermochemistry” ppt download Standard Heat Of Formation Example The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Standard Heats of Reaction PowerPoint Presentation, free download Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. By the way, standard enthalpies of various substances. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Standard enthalpy of. Standard Heat Of Formation Example.
From duanerafanan.blogspot.com
DUANE HESS'S LAW Standard Heat Of Formation Example By the way, standard enthalpies of various substances. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is. Standard Heat Of Formation Example.
From slideplayer.com
Heat in Changes of State and Calculating Heat of Reaction ppt download Standard Heat Of Formation Example For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature. Standard Heat Of Formation Example.
From www.youtube.com
Standard Heat of Formation YouTube Standard Heat Of Formation Example For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. By the way, standard enthalpies of various substances. 193 rows in chemistry. Standard Heat Of Formation Example.
From slideplayer.com
Chapter ppt download Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy. Standard Heat Of Formation Example.
From www.chegg.com
Solved The standard heat of formation, ΔHf, is defined as Standard Heat Of Formation Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. By the way, standard enthalpies of various substances. The standard enthalpy of formation of any element in. Standard Heat Of Formation Example.
From www.chegg.com
Solved The standard heat of formation, ДНі , is defined as Standard Heat Of Formation Example For example, here is a link to obtain the standard enthalpy of formation for carbon dioxide. Standard enthalpy of formation (or heat of formation), δhof, is the enthalpy change when 1 mol of the substance is formed from its constituent elements in their standard states. The standard enthalpy of formation of any element in its standard state is zero by. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2202556 Standard Heat Of Formation Example The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ),. Standard Heat Of Formation Example.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Example The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. 193 rows in chemistry and thermodynamics, the. Standard Heat Of Formation Example.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. By the way, standard enthalpies of various substances. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. The standard enthalpy. Standard Heat Of Formation Example.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Heat Of Formation Example 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, here is. Standard Heat Of Formation Example.
From www.youtube.com
Standard heat of formation problem / Heat of formation formation Standard Heat Of Formation Example The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. For example, although oxygen can exist as ozone (o. Standard Heat Of Formation Example.
From lessonsybil.z5.web.core.windows.net
How To Calculate The Heat Of Formation Standard Heat Of Formation Example For example, although oxygen can exist as ozone (o 3 ), atomic oxygen. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of a pure element is in its reference form. Standard Heat Of Formation Example.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Heat Of Formation Example The standard enthalpy of formation of a pure element is in its reference form its standard enthalpy formation is zero. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone. Standard Heat Of Formation Example.