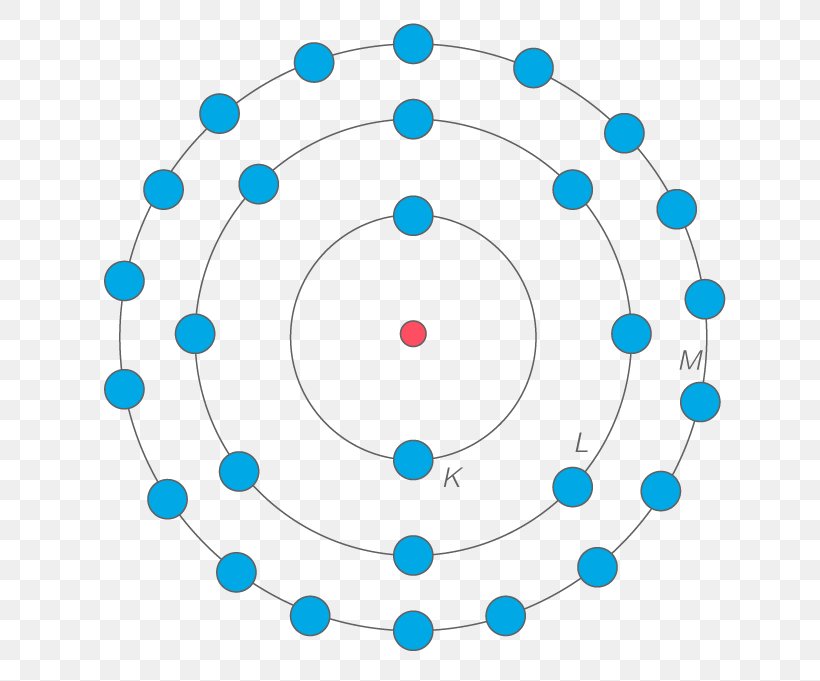
Copper Electrons Shells . In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The energy levels of copper can. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. Copper ion (cu +, cu 2+) electron configuration. First, recall that the n = 3 shell is the first shell. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity.
from diagramlibraryschemer.z19.web.core.windows.net
First, recall that the n = 3 shell is the first shell. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper ion (cu +, cu 2+) electron configuration. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The energy levels of copper can.
Electron Shell Diagram For Copper
Copper Electrons Shells First, recall that the n = 3 shell is the first shell. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. Copper ion (cu +, cu 2+) electron configuration. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. First, recall that the n = 3 shell is the first shell. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The energy levels of copper can. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Electrons Shells How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s. Copper Electrons Shells.
From diagramlibraryschemer.z19.web.core.windows.net
Electron Shell Diagram For Copper Copper Electrons Shells How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In this article, we will explore the electron orbital arrangement,. Copper Electrons Shells.
From www.sciencephoto.com
Copper, atomic structure Stock Image C018/3710 Science Photo Library Copper Electrons Shells Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p.. Copper Electrons Shells.
From www.youtube.com
Copper Electron Configuration Organic Chemistry Examples YouTube Copper Electrons Shells How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper’s electron distribution in different energy levels or shells determines. Copper Electrons Shells.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Electrons Shells Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The electron. Copper Electrons Shells.
From www.earthdate.org
Copper’s Superpower EarthDate Copper Electrons Shells Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The energy levels of copper can. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. First, recall that the n = 3 shell is the first shell. How to write the electron configuration for copper. Copper Electrons Shells.
From algaeplanet.com
University of Basel Discovery Reveals How Diatoms Capture CO₂ so Copper Electrons Shells The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. In this article, we. Copper Electrons Shells.
From www.msn.com
Bismuth is so strongly repelled from it levitates. How? Copper Electrons Shells Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. The energy levels of copper can. Copper ion (cu +, cu 2+) electron configuration. First, recall that the n = 3 shell is the first shell. The electron. Copper Electrons Shells.
From scitechdaily.com
Diatoms Unlock Nature’s Secret to Massive CO2 Capture Copper Electrons Shells The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper ion (cu +, cu 2+) electron configuration. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. Transition elements have electrons in the d orbital, which. Copper Electrons Shells.
From www.technocrazed.com
2.3 Valence and Crystal Structure Copper Electrons Shells In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. First, recall that the n = 3 shell is the first shell. The electron distribution. Copper Electrons Shells.
From favpng.com
Bohr Model Atom Electron Shell Copper, PNG, 512x512px, Bohr Model, Area Copper Electrons Shells How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p.. Copper Electrons Shells.
From izayahmeowcrosby.blogspot.com
Electronic Configuration of Copper Copper Electrons Shells Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. How to write the electron configuration for copper (cu,. Copper Electrons Shells.
From pubs.rsc.org
Regulating NO 2 adsorption at ambient temperature by manipulating Copper Electrons Shells First, recall that the n = 3 shell is the first shell. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order. Copper Electrons Shells.
From brainly.in
Why valency of Scandium is 3 but in google electronic configuration is Copper Electrons Shells Copper ion (cu +, cu 2+) electron configuration. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. Copper’s electron distribution in different energy levels or shells determines its. Copper Electrons Shells.
From www.alamy.com
Symbol and electron diagram for Copper illustration Stock Vector Image Copper Electrons Shells The energy levels of copper can. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the first shell. Copper ion (cu +, cu 2+) electron configuration. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. The electron. Copper Electrons Shells.
From www.wou.edu
Ch105 Chapter 2 Atoms, Elements and The Periodic Table Chemistry Copper Electrons Shells In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. The energy levels of copper can. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the. Copper Electrons Shells.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Periodic Table Element Comparison Compare Copper vs Aluminium Copper Electrons Shells The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. Transition elements have electrons in the d orbital, which. Copper Electrons Shells.
From www.alamy.com
3d render of atom structure of copper isolated over white background Copper Electrons Shells Copper ion (cu +, cu 2+) electron configuration. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The energy levels of copper can. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Copper’s electron distribution. Copper Electrons Shells.
From valenceelectrons.com
How to Find the Valence Electrons for Cobalt (Co)? Copper Electrons Shells Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. First, recall that the n = 3 shell is the first shell. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Transition elements have electrons in the d orbital, which. Copper Electrons Shells.
From www.pngwing.com
Bohr model Electron shell Copper Atom Valence electron, cobalt Copper Electrons Shells First, recall that the n = 3 shell is the first shell. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. The energy levels of copper can. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. The ground state electron configuration of copper is. Copper Electrons Shells.
From www.verticallearning.org
Grade 7 Vertical Science Copper Electrons Shells Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. First, recall that the n = 3 shell is the first shell. Copper’s electron distribution in different energy levels or shells determines its atomic structure and. Copper Electrons Shells.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Electrons Shells The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. Copper ion (cu +, cu 2+) electron configuration. First, recall that the n = 3 shell is the first shell. The energy levels of copper can. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the. Copper Electrons Shells.
From www.nutsvolts.com
Which Way Does Current Really Flow? Nuts & Volts Magazine For The Copper Electrons Shells The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to. Copper Electrons Shells.
From www.sciencephoto.com
Copper, atomic structure Stock Image C013/1552 Science Photo Library Copper Electrons Shells Copper ion (cu +, cu 2+) electron configuration. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the first shell. How to write the. Copper Electrons Shells.
From www.webelements.com
Elements Periodic Table » Copper » properties of free atoms Copper Electrons Shells The energy levels of copper can. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. In this article, we will explore the electron orbital arrangement, shell. Copper Electrons Shells.
From en-academic.com
Copper Copper Electrons Shells How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the first shell. The ground state electron configuration. Copper Electrons Shells.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Electrons Shells How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. First, recall that the n = 3 shell is the first shell. The energy levels. Copper Electrons Shells.
From gioqhhhrg.blob.core.windows.net
What Is The Lewis Structure Of Copper at Corey Savage blog Copper Electrons Shells The energy levels of copper can. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. Copper ion (cu +,. Copper Electrons Shells.
From sciencenotes.org
Copper Facts Cu or Atomic Number 29 Copper Electrons Shells First, recall that the n = 3 shell is the first shell. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. The ground state electron configuration of copper is 1s 2 2s 2. Copper Electrons Shells.
From www.goodwholefood.com
Copper Signs of Copper Deficiency Good Whole Food Copper Electrons Shells Copper ion (cu +, cu 2+) electron configuration. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d orbitals. In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. First, recall that the n = 3 shell is. Copper Electrons Shells.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Electrons Shells Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. Copper ion (cu +, cu 2+) electron configuration. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. The energy levels of copper can. In this article, we will explore the electron orbital arrangement, shell configuration,. Copper Electrons Shells.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free Copper Electrons Shells In this article, we will explore the electron orbital arrangement, shell configuration, and valence electron distribution of copper ions. The energy levels of copper can. First, recall that the n = 3 shell is the first shell. The electron distribution in copper spans across the k, l, m, and n shells, with valence electrons located in the 4s and 3d. Copper Electrons Shells.
From gioqhhhrg.blob.core.windows.net
What Is The Lewis Structure Of Copper at Corey Savage blog Copper Electrons Shells Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. Transition elements have electrons in the d orbital, which introduces some additional nuance in the electron configurations. First, recall that the n = 3 shell is the first shell. The energy levels of copper can. The ground state electron configuration of copper is 1s 2. Copper Electrons Shells.
From periodictable.me
Copper Electron Configuration (Cu) with Orbital Diagram Copper Electrons Shells Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. The ground state electron configuration of copper is 1s 2 2s 2 2p 6 3s 2 3p. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. In this. Copper Electrons Shells.
From valenceelectrons.com
How to Find the Valence Electrons for Selenium (Se)? Copper Electrons Shells Copper ion (cu +, cu 2+) electron configuration. How to write the electron configuration for copper (cu, cu+, and cu2+) in order to write the copper electron configuration we first need to know the. The energy levels of copper can. Copper’s electron distribution in different energy levels or shells determines its atomic structure and reactivity. The ground state electron configuration. Copper Electrons Shells.