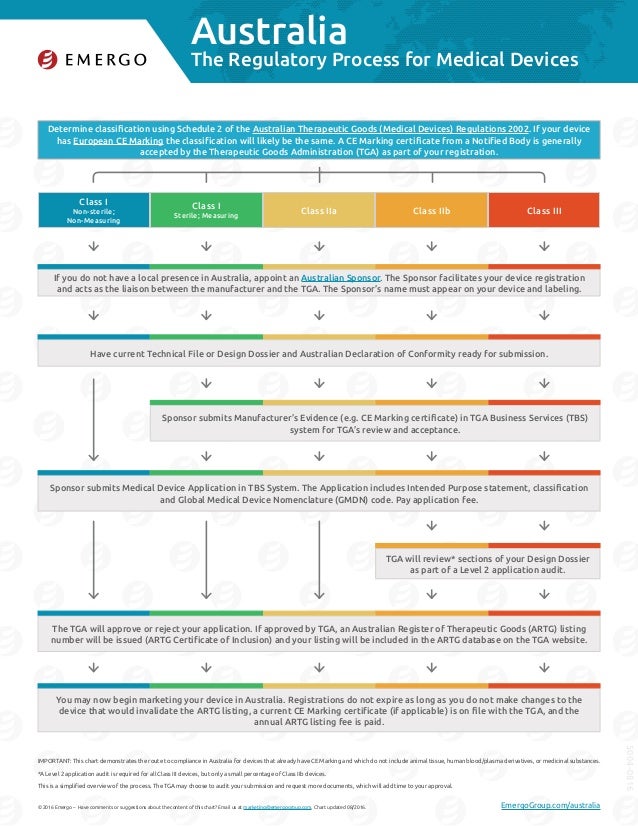
Medical Devices Registration Australia . How to register your medical device in australia. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Appoint an australian sponsor to manage your device. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. For example, medical gloves, bandages, syringes, blood pressure monitors,. Determine the classification of your device according to the australian classification rules. The argmd provides information on the import, export and supply of medical devices within australia. Medical devices include a wide range of products. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body.
from www.slideshare.net
Appoint an australian sponsor to manage your device. Medical devices include a wide range of products. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. They include things like surgical equipment, syringes, gloves, pacemakers, baby. The argmd provides information on the import, export and supply of medical devices within australia. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. For example, medical gloves, bandages, syringes, blood pressure monitors,.
Australia medical device registration and approval process EMERGO
Medical Devices Registration Australia How to register your medical device in australia. The argmd provides information on the import, export and supply of medical devices within australia. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. How to register your medical device in australia. Determine the classification of your device according to the australian classification rules. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. Medical devices include a wide range of products. Appoint an australian sponsor to manage your device. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. For example, medical gloves, bandages, syringes, blood pressure monitors,. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods.
From www.slideshare.net
The regulation of medical devices in Australia Medical Devices Registration Australia Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. For example, medical gloves, bandages, syringes, blood pressure monitors,. Medical devices include a wide range of products. Appoint an australian sponsor to manage your device. How to register your medical device in australia.. Medical Devices Registration Australia.
From www.freyrsolutions.com
Medical Devices and CE Marking Process under the EU MDR Freyr Medical Devices Registration Australia They include things like surgical equipment, syringes, gloves, pacemakers, baby. How to register your medical device in australia. Medical devices include a wide range of products. Determine the classification of your device according to the australian classification rules. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. For example, medical gloves, bandages, syringes, blood pressure monitors,.. Medical Devices Registration Australia.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Medical Devices Registration Australia How to register your medical device in australia. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Appoint an australian sponsor to manage your device. For example, medical gloves,. Medical Devices Registration Australia.
From www.cisema.com
China medical device registration electronic certificates launched Medical Devices Registration Australia How to register your medical device in australia. Appoint an australian sponsor to manage your device. Determine the classification of your device according to the australian classification rules. They include things like surgical equipment, syringes, gloves, pacemakers, baby. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Manufacturers seeking artg inclusion. Medical Devices Registration Australia.
From credevo.com
Medical Device Registration Process in Singapore Credevo Articles Medical Devices Registration Australia Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. The argmd provides information on the import, export and supply of medical devices within australia. Determine the classification of your device according to the australian classification rules. Medical devices include a wide range. Medical Devices Registration Australia.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Medical Devices Registration Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. Medical devices include a wide range of products. Determine the. Medical Devices Registration Australia.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Medical Devices Registration Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Determine the classification of your device according to the australian classification rules. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. For example, medical gloves, bandages, syringes, blood pressure. Medical Devices Registration Australia.
From legalspot8.wordpress.com
Medical Devices Registration Ensuring Safety and Quality in Healthcare Medical Devices Registration Australia They include things like surgical equipment, syringes, gloves, pacemakers, baby. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. Determine the classification of your device according to the australian classification rules. Before any medical device can be supplied in australia, the device. Medical Devices Registration Australia.
From www.regdesk.co
HSA Guidance on Medical Device Product Registration Additional Aspects Medical Devices Registration Australia Appoint an australian sponsor to manage your device. How to register your medical device in australia. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Medical devices include a wide range of products. For example, medical gloves, bandages, syringes, blood pressure monitors,. Before any medical device can be supplied in australia,. Medical Devices Registration Australia.
From www.corpseed.com
CDSCO Registration for Ophthalmic Medical Devices Corpseed Medical Devices Registration Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Appoint an. Medical Devices Registration Australia.
From www.sphericalinsights.com
Australia Reprocessed Medical Devices Market Size, Forecast 2032 Medical Devices Registration Australia How to register your medical device in australia. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. Medical devices include a wide range of products. Determine the classification of your device according to the australian classification rules. For example,. Medical Devices Registration Australia.
From credevo.com
Regulations For Medical Device Approval in Australia Credevo Articles Medical Devices Registration Australia For example, medical gloves, bandages, syringes, blood pressure monitors,. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. Determine the classification of your device according to the australian classification rules. They include things like surgical equipment, syringes, gloves, pacemakers,. Medical Devices Registration Australia.
From www.pacificbridgemedical.com
China Class 2 Device Registration Process & Timeline Chart Medical Devices Registration Australia The argmd provides information on the import, export and supply of medical devices within australia. Medical devices include a wide range of products. Determine the classification of your device according to the australian classification rules. Appoint an australian sponsor to manage your device. For example, medical gloves, bandages, syringes, blood pressure monitors,. Registering a medical device in australia is a. Medical Devices Registration Australia.
From medicaldevices.freyrsolutions.com
Medical Device Registration in India for Foreign Manufactures Freyr Medical Devices Registration Australia How to register your medical device in australia. They include things like surgical equipment, syringes, gloves, pacemakers, baby. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Appoint an australian sponsor to manage your device. Registering a medical. Medical Devices Registration Australia.
From operonstrategist.com
Medical Device Registration in Australia (StepbyStep Guidance Medical Devices Registration Australia For example, medical gloves, bandages, syringes, blood pressure monitors,. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. Medical devices include a wide range of products. The argmd provides information on the import, export and supply of medical devices. Medical Devices Registration Australia.
From www.corpseed.com
CDSCO Registration for Oncology Medical Devices Corpseed Medical Devices Registration Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. Medical devices include a wide range of products. Determine the classification of your device according to the australian classification rules. How to register your medical device in. Medical Devices Registration Australia.
From www.youtube.com
Registration Certificate for the Sale or Distribution of Medical Medical Devices Registration Australia They include things like surgical equipment, syringes, gloves, pacemakers, baby. Appoint an australian sponsor to manage your device. Determine the classification of your device according to the australian classification rules. How to register your medical device in australia. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. The australian. Medical Devices Registration Australia.
From www.joharidigital.com
Health Canada Approval Process for Medical Devices StepbyStep Guide Medical Devices Registration Australia Medical devices include a wide range of products. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. How to register your medical device in australia. Determine the classification of your device according to the australian classification rules. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment. Medical Devices Registration Australia.
From dokumen.tips
(PDF) Regulation of Medical Devices' Registration in Indonesia Medical Devices Registration Australia The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Medical devices include a wide range of products. Appoint an australian sponsor to manage your device. Before any medical device can be supplied in australia, the device must be included in the. Medical Devices Registration Australia.
From www.regdesk.co
HSA Guidance on Medical Device Product Registration Class C and D Medical Devices Registration Australia Determine the classification of your device according to the australian classification rules. Appoint an australian sponsor to manage your device. How to register your medical device in australia. Medical devices include a wide range of products. The argmd provides information on the import, export and supply of medical devices within australia. For example, medical gloves, bandages, syringes, blood pressure monitors,.. Medical Devices Registration Australia.
From www.slideshare.net
Australia medical device registration and approval process EMERGO Medical Devices Registration Australia Medical devices include a wide range of products. Appoint an australian sponsor to manage your device. How to register your medical device in australia. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. The argmd provides information on the. Medical Devices Registration Australia.
From globalregulatorypartners.com
Medical Device Registration in South Korea Medical Devices Registration Australia Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Determine the classification of your device according to the australian classification rules. Medical devices include a wide range of products. Appoint an australian sponsor to manage your device. The australian register of therapeutic goods (artg) is the. Medical Devices Registration Australia.
From www.vrogue.co
Australia Tga Approval Process For Medical Devices vrogue.co Medical Devices Registration Australia Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. How to register your medical device in australia. Medical devices. Medical Devices Registration Australia.
From www.corpseed.com
CDSCO Registration for Software Medical Devices Medical Devices Registration Australia They include things like surgical equipment, syringes, gloves, pacemakers, baby. The argmd provides information on the import, export and supply of medical devices within australia. How to register your medical device in australia. Appoint an australian sponsor to manage your device. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Medical. Medical Devices Registration Australia.
From operonstrategist.com
GMP Certificate for Medical Devices (Standards and Requirements Medical Devices Registration Australia For example, medical gloves, bandages, syringes, blood pressure monitors,. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Medical devices include a wide range of products. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated. Medical Devices Registration Australia.
From morulaa.com
FAQ about Medical Device Registration in India Medical Devices Registration Australia Determine the classification of your device according to the australian classification rules. For example, medical gloves, bandages, syringes, blood pressure monitors,. How to register your medical device in australia. Appoint an australian sponsor to manage your device. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which. Medical Devices Registration Australia.
From dokumen.tips
(PDF) Registration of Medical Devices in India DOKUMEN.TIPS Medical Devices Registration Australia They include things like surgical equipment, syringes, gloves, pacemakers, baby. The argmd provides information on the import, export and supply of medical devices within australia. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other. Medical Devices Registration Australia.
From certificationbody.com.au
TGA Registration Certification Body Australia (CBA) Medical Devices Registration Australia Medical devices include a wide range of products. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. They include things like surgical equipment, syringes,. Medical Devices Registration Australia.
From www.artixio.com
FAQ Singapore (HSA) Regulations for Medical Device Registration Medical Devices Registration Australia How to register your medical device in australia. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the. Medical Devices Registration Australia.
From cmsmedtech.com
MEDICAL DEVICE REGISTRATION IN AUSTRALIA CMS MedTech Medical Devices Registration Australia Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. For example, medical gloves, bandages, syringes, blood pressure monitors,. Appoint an australian sponsor to manage your device. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. Manufacturers seeking artg inclusion will. Medical Devices Registration Australia.
From www.123formbuilder.com
Medical Devices Registration Form Template 123FormBuilder Medical Devices Registration Australia Medical devices include a wide range of products. For example, medical gloves, bandages, syringes, blood pressure monitors,. Registering a medical device in australia is a complex but crucial process that ensures the safety and performance of devices. How to register your medical device in australia. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Appoint an australian sponsor to. Medical Devices Registration Australia.
From www.regdesk.co
MHRA Guidance on Registration of Medical Devices RegDesk Medical Devices Registration Australia Manufacturers seeking artg inclusion will need to provide evidence of acceptable certification from the tga or other conformity assessment body (cab), such as a european notified body. Determine the classification of your device according to the australian classification rules. Medical devices include a wide range of products. How to register your medical device in australia. They include things like surgical. Medical Devices Registration Australia.
From www.cekindo.vn
Understand Medical Devices Registration in Vietnam Medical Devices Registration Australia They include things like surgical equipment, syringes, gloves, pacemakers, baby. Medical devices can be used to diagnose, prevent, treat and monitor medical conditions. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally. The argmd provides information on the import, export and supply of medical devices within australia. How to register your. Medical Devices Registration Australia.
From www.qualtechs.com
QT ANALYSIS AUSTRALIA's MEDICAL DEVICE MARKET AND REGISTRATION Medical Devices Registration Australia Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods (artg), which is regulated by the australian therapeutic goods. Determine the classification of your device according to the australian classification rules. The australian register of therapeutic goods (artg) is the central database of therapeutic goods that can be legally.. Medical Devices Registration Australia.
From operonstrategist.com
Medical Device Registration in Australia (StepbyStep Guidance Medical Devices Registration Australia Appoint an australian sponsor to manage your device. Determine the classification of your device according to the australian classification rules. For example, medical gloves, bandages, syringes, blood pressure monitors,. They include things like surgical equipment, syringes, gloves, pacemakers, baby. Before any medical device can be supplied in australia, the device must be included in the australian register of therapeutic goods. Medical Devices Registration Australia.