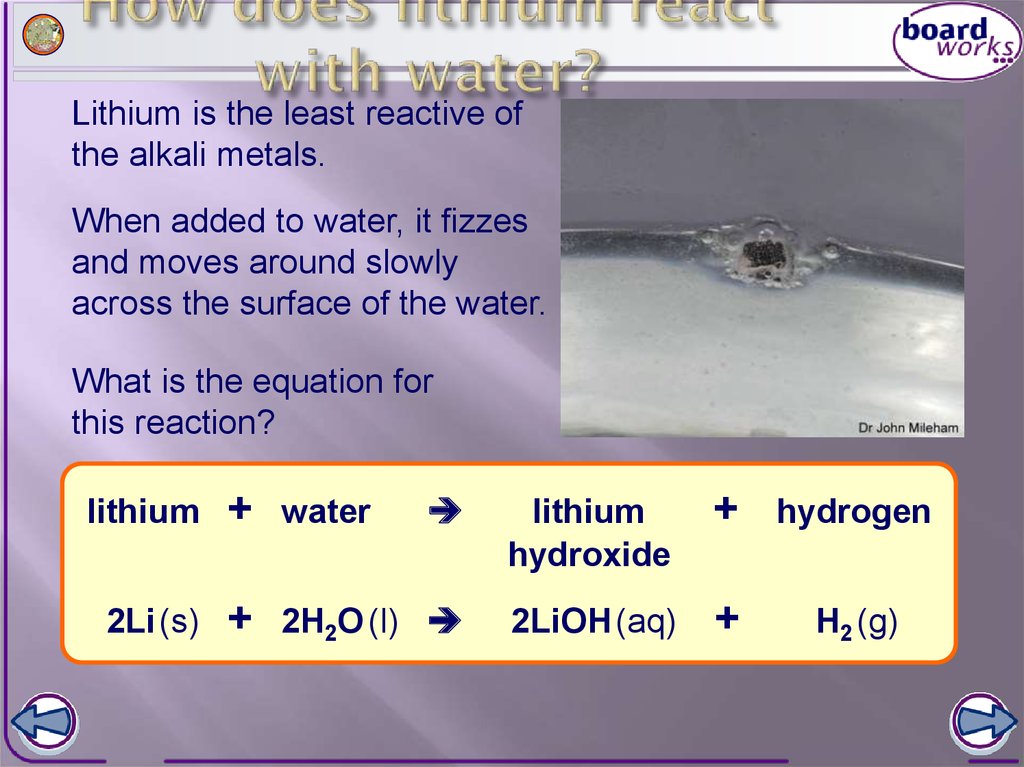
Does Lithium Produce Flame In Water . This video shows the physical properties of li metal and. Part of ncssm core collection: When lithium comes into contact with water, a violent reaction occurs. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. When lithium is added to water, lithium floats. Lithium and its salts turn flames a carmine red color. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. How does lithium react with water? In what way and in what form does lithium react with water? It fizzes steadily and becomes smaller, until it eventually disappears.
from ppt-online.org
Part of ncssm core collection: How does lithium react with water? In what way and in what form does lithium react with water? Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. Lithium and its salts turn flames a carmine red color. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. This video shows the physical properties of li metal and. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. It fizzes steadily and becomes smaller, until it eventually disappears. When lithium is added to water, lithium floats.
The alkali metals презентация онлайн
Does Lithium Produce Flame In Water In what way and in what form does lithium react with water? Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. When lithium is added to water, lithium floats. In what way and in what form does lithium react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. This video shows the physical properties of li metal and. When lithium comes into contact with water, a violent reaction occurs. Part of ncssm core collection: How does lithium react with water? It fizzes steadily and becomes smaller, until it eventually disappears. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. Lithium and its salts turn flames a carmine red color.
From keplarllp.com
😀 Ammonium nitrate lab. Ammonium Nitrate. 20190211 Does Lithium Produce Flame In Water Lithium and its salts turn flames a carmine red color. It fizzes steadily and becomes smaller, until it eventually disappears. This video shows the physical properties of li metal and. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. How does lithium react with water? Part of ncssm core collection:. Does Lithium Produce Flame In Water.
From sciencenotes.org
Colored Fire Spray Bottles Does Lithium Produce Flame In Water Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. It fizzes steadily and becomes smaller, until it eventually disappears. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. When lithium is added to water, lithium floats. In what way and in what form does lithium react with water?. Does Lithium Produce Flame In Water.
From hxejdawix.blob.core.windows.net
Gas Flame Is Yellow Not Blue at Essie Barber blog Does Lithium Produce Flame In Water How does lithium react with water? When lithium is added to water, lithium floats. Lithium and its salts turn flames a carmine red color. This video shows the physical properties of li metal and. It fizzes steadily and becomes smaller, until it eventually disappears. Part of ncssm core collection: When lithium comes into contact with water, a violent reaction occurs.. Does Lithium Produce Flame In Water.
From www.youtube.com
Burning Lithium in Water Close Up YouTube Does Lithium Produce Flame In Water It fizzes steadily and becomes smaller, until it eventually disappears. This video shows the physical properties of li metal and. In what way and in what form does lithium react with water? Part of ncssm core collection: How does lithium react with water? When lithium comes into contact with water, a violent reaction occurs. [wikimedia] this qualitative reaction for lithium. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Lithium Flame Does Lithium Produce Flame In Water Part of ncssm core collection: Lithium and its salts turn flames a carmine red color. In what way and in what form does lithium react with water? When lithium is added to water, lithium floats. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react. Does Lithium Produce Flame In Water.
From lessonlibminimalist.z13.web.core.windows.net
Science Experiments Using Fire Does Lithium Produce Flame In Water Lithium and its salts turn flames a carmine red color. How does lithium react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In what way and in what form does lithium react with water? Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. When lithium. Does Lithium Produce Flame In Water.
From www.youtube.com
What happens when Lithium burns? YouTube Does Lithium Produce Flame In Water It fizzes steadily and becomes smaller, until it eventually disappears. In what way and in what form does lithium react with water? When lithium comes into contact with water, a violent reaction occurs. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. Part of ncssm. Does Lithium Produce Flame In Water.
From fineartamerica.com
Lithium Flame Test Photograph by Science Photo Library Fine Art America Does Lithium Produce Flame In Water When lithium is added to water, lithium floats. In what way and in what form does lithium react with water? How does lithium react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. It fizzes steadily and becomes smaller, until it eventually disappears. Part of ncssm core collection:. Does Lithium Produce Flame In Water.
From mammothmemory.net
Lithium reaction the sulphuric acid is violent but longer Does Lithium Produce Flame In Water When lithium comes into contact with water, a violent reaction occurs. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. How does lithium react with water? In what way and in what form does lithium react with water? Part of ncssm core collection: When lithium is added to water, lithium. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Lithium Flame Does Lithium Produce Flame In Water When lithium is added to water, lithium floats. How does lithium react with water? This video shows the physical properties of li metal and. Lithium and its salts turn flames a carmine red color. It fizzes steadily and becomes smaller, until it eventually disappears. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. In what way and in. Does Lithium Produce Flame In Water.
From www.youtube.com
Burning lithium metal in pure oxygen! YouTube Does Lithium Produce Flame In Water How does lithium react with water? This video shows the physical properties of li metal and. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. When lithium comes into contact with water, a violent reaction occurs. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. Lithium and its. Does Lithium Produce Flame In Water.
From in.pinterest.com
Learn how to make colored fire at home in your fireplace or campfire Does Lithium Produce Flame In Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Part of ncssm core collection: Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. In what way and in what form does lithium react with water? When lithium comes into contact with water, a violent reaction occurs. It fizzes. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Lithium Battery Explosion Does Lithium Produce Flame In Water It fizzes steadily and becomes smaller, until it eventually disappears. Lithium and its salts turn flames a carmine red color. Part of ncssm core collection: All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. When lithium comes into contact with water, a violent reaction occurs. [wikimedia] this qualitative reaction for. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Lithium Flame Does Lithium Produce Flame In Water When lithium is added to water, lithium floats. Lithium and its salts turn flames a carmine red color. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. When lithium comes into contact with water, a violent reaction occurs. In what way and in what form does lithium react with water? This video shows the physical properties. Does Lithium Produce Flame In Water.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Does Lithium Produce Flame In Water [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. In what way and in what form does lithium react with water? It fizzes steadily and becomes smaller, until it eventually disappears. How does lithium react with water? Lithium and its salts turn flames a carmine. Does Lithium Produce Flame In Water.
From ballardsharon.blogspot.com
Why Do Some Elements Produce Colorful Flames Ballard Sharon Does Lithium Produce Flame In Water Lithium and its salts turn flames a carmine red color. When lithium comes into contact with water, a violent reaction occurs. When lithium is added to water, lithium floats. This video shows the physical properties of li metal and. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. Part of ncssm core collection: How does lithium. Does Lithium Produce Flame In Water.
From www.youtube.com
Experiment 5 colored flames (How to color fire with salts) YouTube Does Lithium Produce Flame In Water This video shows the physical properties of li metal and. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Part of ncssm core collection: Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. In what way and in what form does lithium react with water? [wikimedia] this qualitative. Does Lithium Produce Flame In Water.
From fineartamerica.com
Lithium Flame Test Photograph by Science Photo Library Fine Art America Does Lithium Produce Flame In Water [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. Part of ncssm core collection: When lithium is added to water, lithium floats. In what way and. Does Lithium Produce Flame In Water.
From chemicaldb.netlify.app
Lithium in water explosion Does Lithium Produce Flame In Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Part of ncssm core collection: [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. How does lithium react with water? When lithium is added to water, lithium floats. In what way and in what form does lithium. Does Lithium Produce Flame In Water.
From wjec-chemistry-c3-revision.blogspot.com
WJEC Chemistry C2 online revision Reactions of alkali metals and halogens Does Lithium Produce Flame In Water Lithium and its salts turn flames a carmine red color. This video shows the physical properties of li metal and. When lithium is added to water, lithium floats. When lithium comes into contact with water, a violent reaction occurs. Part of ncssm core collection: In what way and in what form does lithium react with water? Lithium reacts intensely with. Does Lithium Produce Flame In Water.
From mammothmemory.net
When metals are heated it reacts with oxygen to create flame Does Lithium Produce Flame In Water When lithium is added to water, lithium floats. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. In what way and in what form does lithium react with water? Lithium and its salts turn flames a carmine red color. How does lithium react with water? When lithium comes into contact with water, a violent reaction occurs.. Does Lithium Produce Flame In Water.
From www.flowvis.org
A fire whirl mixes flames from methanol doped with lithium to make red Does Lithium Produce Flame In Water This video shows the physical properties of li metal and. Part of ncssm core collection: In what way and in what form does lithium react with water? Lithium and its salts turn flames a carmine red color. When lithium comes into contact with water, a violent reaction occurs. It fizzes steadily and becomes smaller, until it eventually disappears. When lithium. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Lithium Flame Does Lithium Produce Flame In Water Part of ncssm core collection: When lithium is added to water, lithium floats. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. How does lithium react with water? This video shows the physical properties of li metal and. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. [wikimedia]. Does Lithium Produce Flame In Water.
From www.nist.gov
Difference Between a Flame in Gravity and Microgravity Does Lithium Produce Flame In Water Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. This video shows the physical properties of li metal and. Lithium and its salts turn flames a carmine red color. Part of ncssm core collection: When lithium is added to water, lithium floats. How does lithium. Does Lithium Produce Flame In Water.
From proper-cooking.info
Lithium Chloride Flame Color Does Lithium Produce Flame In Water Lithium and its salts turn flames a carmine red color. It fizzes steadily and becomes smaller, until it eventually disappears. When lithium comes into contact with water, a violent reaction occurs. When lithium is added to water, lithium floats. How does lithium react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even. Does Lithium Produce Flame In Water.
From www.alamy.com
A flame test for lithium. Lithium's compounds produce a red coloured Does Lithium Produce Flame In Water Lithium and its salts turn flames a carmine red color. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. This video shows the physical properties of li metal and. Part of ncssm core collection: How does lithium. Does Lithium Produce Flame In Water.
From www.youtube.com
Lithium flame color YouTube Does Lithium Produce Flame In Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. When lithium is added to water, lithium floats. How does lithium react with water? Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. Lithium and its salts turn flames a carmine red color. In what way and in what. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Rubidium Flame Does Lithium Produce Flame In Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. When lithium is added to water, lithium floats. In what way and in what form does lithium react with water? Lithium reacts intensely with water, forming lithium hydroxide. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Lithium And Water Equation Does Lithium Produce Flame In Water When lithium is added to water, lithium floats. This video shows the physical properties of li metal and. How does lithium react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. It fizzes steadily and becomes smaller, until it eventually disappears. Lithium reacts intensely with water, forming lithium. Does Lithium Produce Flame In Water.
From ppt-online.org
The alkali metals презентация онлайн Does Lithium Produce Flame In Water Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. This video shows the physical properties of li metal and. In what way and in what form does lithium react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. Lithium and its salts turn flames a carmine. Does Lithium Produce Flame In Water.
From ar.inspiredpencil.com
Lithium Flame Test Does Lithium Produce Flame In Water [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. It fizzes steadily and becomes smaller, until it eventually disappears. When lithium comes into contact with water, a violent reaction occurs. This video shows the physical properties of li metal and. Part of ncssm core collection: In what way and in what form does lithium react with. Does Lithium Produce Flame In Water.
From www.youtube.com
Qualitative Flame Test LiCl Lithium Chloride YouTube Does Lithium Produce Flame In Water Part of ncssm core collection: [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. In what way and in what form does lithium react with water? How does lithium react with water? When lithium comes into contact with water, a violent reaction occurs. When lithium. Does Lithium Produce Flame In Water.
From www.hindustantimes.com
Lithium ore found in J&K, but more studies needed Latest News India Does Lithium Produce Flame In Water It fizzes steadily and becomes smaller, until it eventually disappears. Part of ncssm core collection: How does lithium react with water? All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. When lithium is added to water, lithium floats. Lithium reacts intensely with water, forming lithium hydroxide and highly flammable. When. Does Lithium Produce Flame In Water.
From blog.nus.edu.sg
Lithiumion battery is the future of renewable green energy but how Does Lithium Produce Flame In Water How does lithium react with water? Part of ncssm core collection: [wikimedia] this qualitative reaction for lithium was established by leopold gmelin in 1818. When lithium is added to water, lithium floats. This video shows the physical properties of li metal and. All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Does Lithium Produce Flame In Water.
From mammothmemory.net
Lithium reacting with water gives off hydrogen gas hydroxide Does Lithium Produce Flame In Water All of group 1 elements —lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold water. In what way and in what form does lithium react with water? When lithium is added to water, lithium floats. It fizzes steadily and becomes smaller, until it eventually disappears. How does lithium react with water? Lithium and its salts turn. Does Lithium Produce Flame In Water.