How To Do A Calorimetry Problem . Solving a basic calorimetry problem. Identify the change in temperature by. It shows you how to. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. This chemistry video tutorial explains how to solve basic calorimetry problems. It discusses how to calculate the heat energy. If the heat capacity of the calorimeter is 21.6. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. This video steps you through one complete calorimetry problem, demonstrating. Identify the mass of the substance and the specific heat capacity constant for the substance. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry.
from www.youtube.com
In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. Identify the change in temperature by. It shows you how to. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Apply the first law of thermodynamics to calorimetry. Solving a basic calorimetry problem. If the heat capacity of the calorimeter is 21.6. Identify the mass of the substance and the specific heat capacity constant for the substance. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. This video steps you through one complete calorimetry problem, demonstrating.
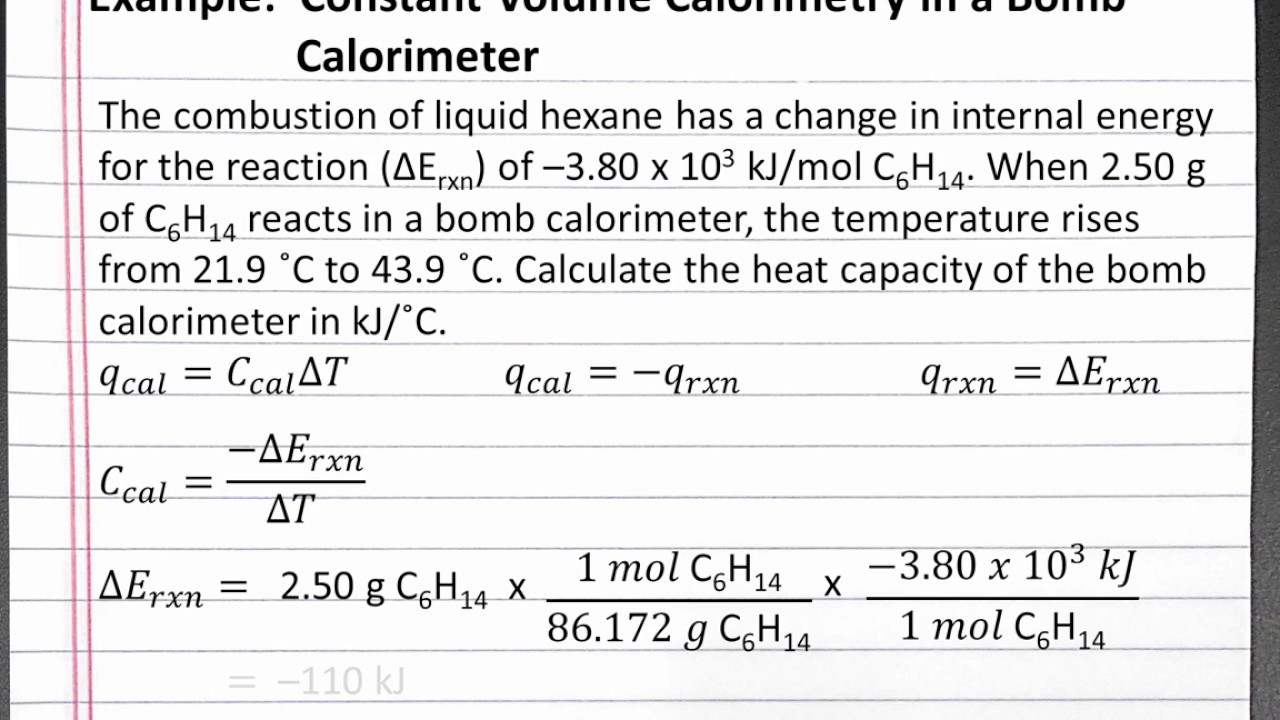
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube
How To Do A Calorimetry Problem When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Apply the first law of thermodynamics to calorimetry. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. If the heat capacity of the calorimeter is 21.6. This chemistry video tutorial explains how to solve basic calorimetry problems. Identify the change in temperature by. This video steps you through one complete calorimetry problem, demonstrating. Identify the mass of the substance and the specific heat capacity constant for the substance. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Solving a basic calorimetry problem. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. It discusses how to calculate the heat energy. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. It shows you how to.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube How To Do A Calorimetry Problem This chemistry video tutorial explains how to solve basic calorimetry problems. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. It shows you how to. This video steps you through one complete calorimetry problem, demonstrating. Identify the mass of the substance and the specific heat capacity constant for the substance. If the heat capacity of the calorimeter. How To Do A Calorimetry Problem.
From worksheetdbroyces.z21.web.core.windows.net
Coffee Cup Calorimetry Practice Problems How To Do A Calorimetry Problem Identify the change in temperature by. This chemistry video tutorial explains how to solve basic calorimetry problems. This video steps you through one complete calorimetry problem, demonstrating. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. If the heat capacity of the calorimeter is 21.6. It shows you how to. When 1.0. How To Do A Calorimetry Problem.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe How To Do A Calorimetry Problem It shows you how to. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Apply the first law of thermodynamics to calorimetry. It discusses how to calculate. How To Do A Calorimetry Problem.
From lessoncampuselkin.z19.web.core.windows.net
Solving A Basic Calorimetry Problem How To Do A Calorimetry Problem Identify the change in temperature by. It discusses how to calculate the heat energy. It shows you how to. Identify the mass of the substance and the specific heat capacity constant for the substance. Solving a basic calorimetry problem. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. In this set of. How To Do A Calorimetry Problem.
From www.chegg.com
Solved GOAL Solve a calorimetry problem involving three How To Do A Calorimetry Problem When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. This chemistry video tutorial explains how to solve basic calorimetry problems. It discusses. How To Do A Calorimetry Problem.
From www.youtube.com
Principle of Calorimetry YouTube How To Do A Calorimetry Problem When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Apply the first law of thermodynamics to calorimetry. Solving a basic calorimetry problem. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. If the heat capacity of the calorimeter is 21.6. It shows you how to. This chemistry video tutorial. How To Do A Calorimetry Problem.
From www.youtube.com
Calorimetry Problem YouTube How To Do A Calorimetry Problem When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Identify the change in temperature by. Compare heat flow from hot to cold. How To Do A Calorimetry Problem.
From www.youtube.com
ALEKS Solving a Basic Calorimetry Problem YouTube How To Do A Calorimetry Problem Solving a basic calorimetry problem. It discusses how to calculate the heat energy. This chemistry video tutorial explains how to solve basic calorimetry problems. Identify the change in temperature by. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. When 1.0 g of fructose, c 6. How To Do A Calorimetry Problem.
From www.youtube.com
General Chemistry II Solving Calorimetry Problems Neutralization How To Do A Calorimetry Problem Identify the mass of the substance and the specific heat capacity constant for the substance. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. This video steps you through one complete calorimetry problem, demonstrating. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits,. How To Do A Calorimetry Problem.
From www.youtube.com
How to find calorimeter constant YouTube How To Do A Calorimetry Problem It discusses how to calculate the heat energy. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. Apply the first law of thermodynamics to calorimetry. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. It shows you how to. When 1.0 g of fructose, c 6. How To Do A Calorimetry Problem.
From mfawriting608.web.fc2.com
How to solve calorimetry problems How To Do A Calorimetry Problem This chemistry video tutorial explains how to solve basic calorimetry problems. Identify the change in temperature by. It discusses how to calculate the heat energy. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. Solving a basic calorimetry problem. In this set of practice questions, we will go over the main types of questions on calorimetry including. How To Do A Calorimetry Problem.
From www.youtube.com
Calorimetry Problem Solving YouTube How To Do A Calorimetry Problem Identify the mass of the substance and the specific heat capacity constant for the substance. Apply the first law of thermodynamics to calorimetry. Solving a basic calorimetry problem. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Compare heat. How To Do A Calorimetry Problem.
From www.vrogue.co
What Is Calorimetry With Pictures vrogue.co How To Do A Calorimetry Problem In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. If the heat capacity of the calorimeter is 21.6. It discusses how to calculate the heat energy. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. This video steps you through one complete calorimetry problem, demonstrating. Solving. How To Do A Calorimetry Problem.
From studylib.net
Problem Calorimetry How To Do A Calorimetry Problem Apply the first law of thermodynamics to calorimetry. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. If the heat capacity of the calorimeter is 21.6. This chemistry video tutorial explains how to solve basic calorimetry problems. It discusses how to calculate the heat energy. Solving a basic calorimetry problem. When 1.0 g of fructose, c 6. How To Do A Calorimetry Problem.
From www.vrogue.co
Chemistry 101 Constant Pressure Calorimetry Youtube vrogue.co How To Do A Calorimetry Problem When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. This chemistry video tutorial explains how to solve basic calorimetry problems. This video steps you through one complete calorimetry problem, demonstrating. Solving a basic calorimetry problem. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in. How To Do A Calorimetry Problem.
From www.youtube.com
Ch 12 Heat Calorimetry Problem Involving A Phase Change YouTube How To Do A Calorimetry Problem This chemistry video tutorial explains how to solve basic calorimetry problems. Solving a basic calorimetry problem. Apply the first law of thermodynamics to calorimetry. Identify the change in temperature by. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. This video steps you through one complete calorimetry problem, demonstrating. It discusses how to calculate the heat energy.. How To Do A Calorimetry Problem.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How To Do A Calorimetry Problem Apply the first law of thermodynamics to calorimetry. This video steps you through one complete calorimetry problem, demonstrating. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Identify the change in temperature by. It discusses how to calculate the heat energy. Compare heat flow from hot to cold objects in an ideal. How To Do A Calorimetry Problem.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo How To Do A Calorimetry Problem Identify the change in temperature by. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. This chemistry video tutorial explains how to solve basic calorimetry problems. It shows you how to. This video steps you through one complete calorimetry problem, demonstrating. This chemistry video tutorial explains how to solve calorimetry problems in. How To Do A Calorimetry Problem.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube How To Do A Calorimetry Problem Identify the mass of the substance and the specific heat capacity constant for the substance. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. Solving a basic calorimetry problem. Apply the first law of thermodynamics to calorimetry. This video. How To Do A Calorimetry Problem.
From materialschreiner.z19.web.core.windows.net
Calorimetry Sample Problems With Solutions How To Do A Calorimetry Problem This video steps you through one complete calorimetry problem, demonstrating. It discusses how to calculate the heat energy. Identify the mass of the substance and the specific heat capacity constant for the substance. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. Identify the change in temperature by. Solving a basic calorimetry problem. This chemistry video tutorial. How To Do A Calorimetry Problem.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 How To Do A Calorimetry Problem Identify the mass of the substance and the specific heat capacity constant for the substance. This video steps you through one complete calorimetry problem, demonstrating. It discusses how to calculate the heat energy. Identify the change in temperature by. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. This chemistry video tutorial explains how. How To Do A Calorimetry Problem.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube How To Do A Calorimetry Problem Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. If the heat capacity of the calorimeter is 21.6. It discusses how to calculate the heat energy. This video steps you through one complete calorimetry problem, demonstrating. Solving a basic calorimetry problem. It shows you how to. Identify the mass of the substance and the. How To Do A Calorimetry Problem.
From ayanahcristien.blogspot.com
20+ Calculating Heat Of Reaction From ConstantPressure Calorimetry How To Do A Calorimetry Problem Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It shows you how to. Identify the mass of the substance and the specific heat capacity constant for the substance. This video steps you through one complete calorimetry problem, demonstrating. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. When 1.00 g. How To Do A Calorimetry Problem.
From www.reddit.com
Help with calorimetry problem… r/ChemistryTeachers How To Do A Calorimetry Problem In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. If the heat capacity of the calorimeter is 21.6. It discusses how to calculate the heat energy. This video steps you through one complete calorimetry problem, demonstrating. Identify the mass of the substance and the specific heat capacity constant for. How To Do A Calorimetry Problem.
From www.youtube.com
Chapter 10 17 PROBLEM Coffee Cup Calorimeter YouTube How To Do A Calorimetry Problem If the heat capacity of the calorimeter is 21.6. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It discusses how to calculate the heat energy. Solving a basic calorimetry problem. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. This chemistry video. How To Do A Calorimetry Problem.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo How To Do A Calorimetry Problem It discusses how to calculate the heat energy. Identify the mass of the substance and the specific heat capacity constant for the substance. Solving a basic calorimetry problem. This video steps you through one complete calorimetry problem, demonstrating. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen. How To Do A Calorimetry Problem.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 How To Do A Calorimetry Problem Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. If the heat capacity of the calorimeter is 21.6. Apply the first law of thermodynamics to calorimetry. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found. How To Do A Calorimetry Problem.
From courses.lumenlearning.com
Calorimetry Chemistry I How To Do A Calorimetry Problem If the heat capacity of the calorimeter is 21.6. This video steps you through one complete calorimetry problem, demonstrating. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. It discusses how to calculate the heat energy. Identify the change in temperature by. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly. How To Do A Calorimetry Problem.
From www.youtube.com
Bomb Calorimetry Problem (Chemistry) YouTube How To Do A Calorimetry Problem Identify the change in temperature by. If the heat capacity of the calorimeter is 21.6. It shows you how to. This chemistry video tutorial explains how to solve basic calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Solving a basic calorimetry problem. It discusses how to calculate the heat energy. Identify. How To Do A Calorimetry Problem.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals How To Do A Calorimetry Problem This video steps you through one complete calorimetry problem, demonstrating. It shows you how to. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. Identify the mass of the substance and the specific heat capacity constant for the substance. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. Solving. How To Do A Calorimetry Problem.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How To Do A Calorimetry Problem It shows you how to. If the heat capacity of the calorimeter is 21.6. When 1.00 g of coal is burned in a bomb calorimeter, the temperature increases by 1.48 °c. This video steps you through one complete calorimetry problem, demonstrating. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. When 1.0 g of. How To Do A Calorimetry Problem.
From www.youtube.com
Lecture 1.13 simple calorimetry problems YouTube How To Do A Calorimetry Problem If the heat capacity of the calorimeter is 21.6. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in oxygen in a bomb calorimeter, the temperature of the. This chemistry video tutorial explains how to solve calorimetry problems in thermochemistry. This video steps you through one complete calorimetry problem,. How To Do A Calorimetry Problem.
From www.youtube.com
Solving Problems Involving Calorimetry YouTube How To Do A Calorimetry Problem This video steps you through one complete calorimetry problem, demonstrating. It shows you how to. If the heat capacity of the calorimeter is 21.6. Identify the change in temperature by. This chemistry video tutorial explains how to solve basic calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. It discusses how to. How To Do A Calorimetry Problem.
From snipe.fm
😍 How to solve calorimetry problems. How to solve calorimetry problems How To Do A Calorimetry Problem If the heat capacity of the calorimeter is 21.6. This chemistry video tutorial explains how to solve basic calorimetry problems. This video steps you through one complete calorimetry problem, demonstrating. Identify the change in temperature by. It shows you how to. It discusses how to calculate the heat energy. Solving a basic calorimetry problem. This chemistry video tutorial explains how. How To Do A Calorimetry Problem.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube How To Do A Calorimetry Problem Solving a basic calorimetry problem. This chemistry video tutorial explains how to solve basic calorimetry problems. If the heat capacity of the calorimeter is 21.6. Apply the first law of thermodynamics to calorimetry. Identify the change in temperature by. When 1.0 g of fructose, c 6 h 12 o 6 (s), a sugar commonly found in fruits, is burned in. How To Do A Calorimetry Problem.