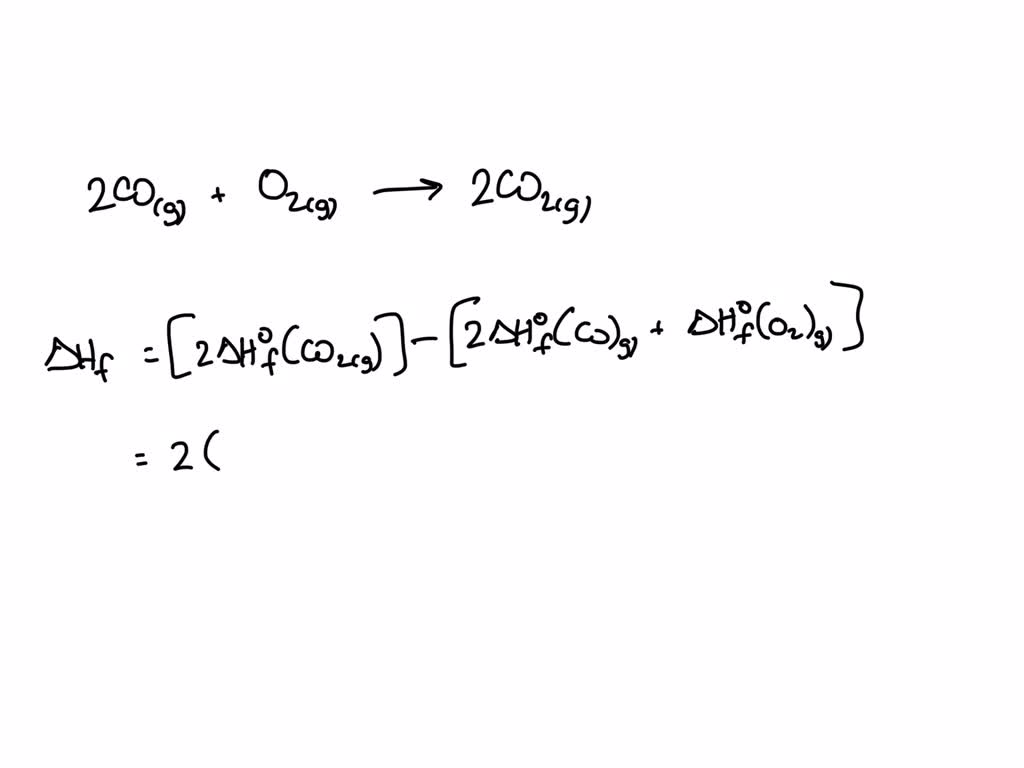Standard Enthalpy Of Formation Reaction With Oxygen . The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The standard enthalpy of formation of any element in its standard state is zero by definition. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. The boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances involved. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
from www.numerade.com
The boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances involved. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. For example, although oxygen can. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g
Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. The boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances involved. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. For example, although oxygen can. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Oxygen Gas Enthalpy Of Formation Standard Enthalpy Of Formation Reaction With Oxygen It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. For example, although oxygen can. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a. Standard Enthalpy Of Formation Reaction With Oxygen.
From exozagkku.blob.core.windows.net
Standard Enthalpy Of Formation Symbol at Diane Downs blog Standard Enthalpy Of Formation Reaction With Oxygen This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25. Standard Enthalpy Of Formation Reaction With Oxygen.
From printablelibmolines.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Reaction With Oxygen For example, although oxygen can. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. A standard. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. It means that 33.2 kj of energy is required to form one mole of no 2 from ½. Standard Enthalpy Of Formation Reaction With Oxygen.
From priaxon.com
What Is Standard Enthalpy Of Formation Of Nh3 Gas Templates Printable Standard Enthalpy Of Formation Reaction With Oxygen The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2). Standard Enthalpy Of Formation Reaction With Oxygen.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation Reaction With Oxygen A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n. Standard Enthalpy Of Formation Reaction With Oxygen.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. For example, although oxygen can. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. The boldfaced values are the coefficients and the other ones are the standard. Standard Enthalpy Of Formation Reaction With Oxygen.
From mungfali.com
Standard Enthalpy Of Formation Equation Standard Enthalpy Of Formation Reaction With Oxygen The boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances involved. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The. Standard Enthalpy Of Formation Reaction With Oxygen.
From ar.inspiredpencil.com
Enthalpy Of Reaction Standard Enthalpy Of Formation Reaction With Oxygen This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard molar enthalpy of formation δh o f is the enthalpy change. Standard Enthalpy Of Formation Reaction With Oxygen.
From mungfali.com
Enthalpies Of Formation Chart Standard Enthalpy Of Formation Reaction With Oxygen 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a Standard Enthalpy Of Formation Reaction With Oxygen 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute. Standard Enthalpy Of Formation Reaction With Oxygen.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Reaction With Oxygen It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is. Standard Enthalpy Of Formation Reaction With Oxygen.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Reaction With Oxygen The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any. Standard Enthalpy Of Formation Reaction With Oxygen.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Standard Enthalpy Of Formation Reaction With Oxygen It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Enthalpy Of Formation Reaction With Oxygen.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. A standard enthalpy of formation $δh°_f$ is. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Enthalpy Of Formation Reaction With Oxygen 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. The standard enthalpy of the formation of nitrogen dioxide is +33.2. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Reaction With Oxygen For example, although oxygen can. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. This equation essentially states that the standard enthalpy change of formation is equal. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Reaction With Oxygen For example, although oxygen can. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution,. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.numerade.com
SOLVED Using the standard enthalpies of formation found in the Standard Enthalpy Of Formation Reaction With Oxygen It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Reaction With Oxygen It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can. This equation. Standard Enthalpy Of Formation Reaction With Oxygen.
From pt.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in. Standard Enthalpy Of Formation Reaction With Oxygen.
From worksheetdbblags.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Standard Enthalpy Of Formation Reaction With Oxygen A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. The boldfaced values are the coefficients and the other ones are the standard enthalpy of formation for the four substances involved. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Reaction for the Standard Enthalpy Of Formation Reaction With Oxygen This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Reaction With Oxygen This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. For example, although oxygen can. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. 193 rows in chemistry and. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.youtube.com
Calculating Reaction Enthalpy from Enthalpies of Formation YouTube Standard Enthalpy Of Formation Reaction With Oxygen For example, although oxygen can. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. It means that 33.2 kj of energy is required to form one mole of no 2. Standard Enthalpy Of Formation Reaction With Oxygen.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This equation essentially states that the standard enthalpy change of formation is equal to the sum. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. For example, although oxygen can. This equation essentially states that the standard enthalpy change of formation is equal. Standard Enthalpy Of Formation Reaction With Oxygen.
From mungfali.com
Standard Enthalpy Change Equation Standard Enthalpy Of Formation Reaction With Oxygen A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance. Standard Enthalpy Of Formation Reaction With Oxygen.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Reaction With Oxygen The standard molar enthalpy of formation δh o f is the enthalpy change when 1 mole of a pure substance, or a 1 m solute concentration in a solution, is. The standard enthalpy of the formation of nitrogen dioxide is +33.2 kj/mol. The standard enthalpy of formation of any element in its standard state is zero by definition. A standard. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Reaction With Oxygen For example, although oxygen can. A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o. Standard Enthalpy Of Formation Reaction With Oxygen.
From oxygengasnaraeru.blogspot.com
Oxygen Gas Enthalpy Of Formation Of Oxygen Gas Standard Enthalpy Of Formation Reaction With Oxygen For example, although oxygen can. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. A standard enthalpy of formation δ h f. Standard Enthalpy Of Formation Reaction With Oxygen.
From maraferstravis.blogspot.com
Standard Enthalpy of Combustion Standard Enthalpy Of Formation Reaction With Oxygen 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure and 25 ˚c.. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Reaction With Oxygen The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can. This equation essentially states that the standard enthalpy change of formation is equal to the sum of the standard enthalpies of formation of the. A standard enthalpy of formation δ h f ° δ h f ° is an. Standard Enthalpy Of Formation Reaction With Oxygen.
From www.nagwa.com
Question Video Calculating the Enthalpy Change for the Reaction Standard Enthalpy Of Formation Reaction With Oxygen A standard enthalpy of formation δ h f ° δ h f ° is an enthalpy change for a reaction in which exactly 1 mole of. It means that 33.2 kj of energy is required to form one mole of no 2 from ½ mole of nitrogen (n 2) and one mole of oxygen (o 2) at 1 atmospheric pressure. Standard Enthalpy Of Formation Reaction With Oxygen.