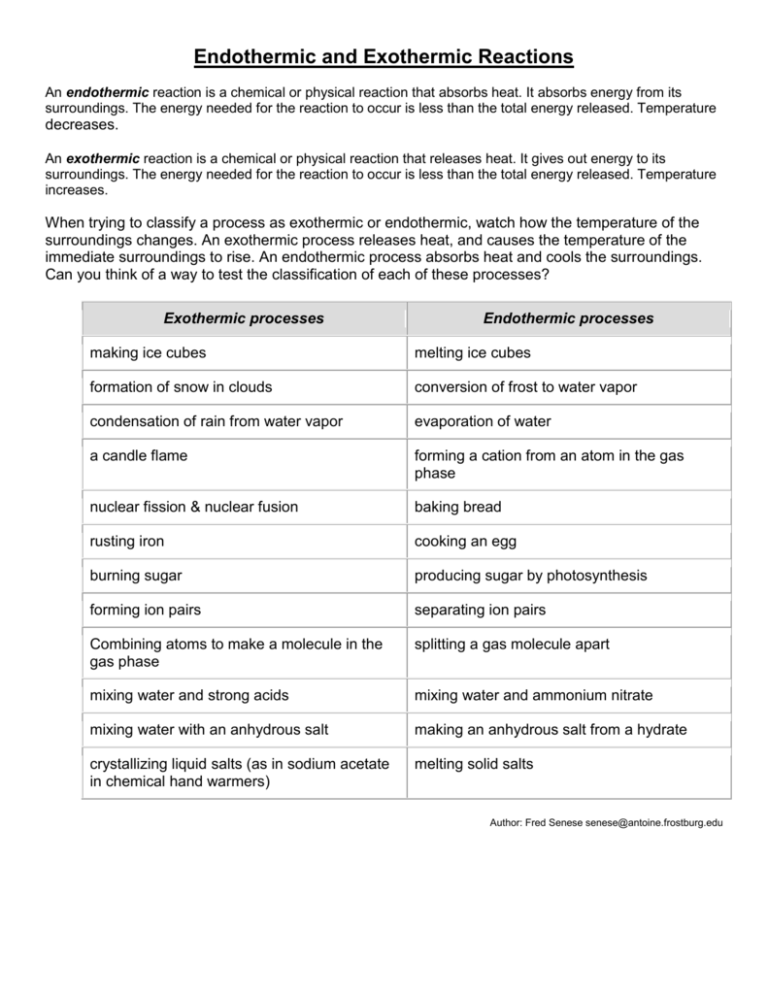
Endothermic Reaction Examples Experiments . An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. You can use these when asked to cite an example or to get ideas to set up a demonstration of an. In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Epsom salt and water create an interesting endothermic chemical reaction. This is an endothermic reaction, which. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. An endothermic reaction is a chemical reaction that absorbs. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. This is what makes it a fun demonstration and effective teaching activity. The reaction between vinegar and bicarbonate soda can be written as: Here's a list of examples of endothermic reactions.
from studyzoneharper.z21.web.core.windows.net
The reaction between vinegar and bicarbonate soda can be written as: An endothermic reaction is a chemical reaction that absorbs. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Epsom salt and water create an interesting endothermic chemical reaction. In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. Here's a list of examples of endothermic reactions. This is an endothermic reaction, which. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride.
Endothermic Vs Exothermic Practice Problems
Endothermic Reaction Examples Experiments Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. You can use these when asked to cite an example or to get ideas to set up a demonstration of an. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Epsom salt and water create an interesting endothermic chemical reaction. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. Here's a list of examples of endothermic reactions. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. This is an endothermic reaction, which. An endothermic reaction is a chemical reaction that absorbs. This is what makes it a fun demonstration and effective teaching activity. In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. The reaction between vinegar and bicarbonate soda can be written as: Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Examples Experiments Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. This is what makes it a fun demonstration and effective teaching activity. The reaction between vinegar and bicarbonate soda can be written as: An endothermic reaction. Endothermic Reaction Examples Experiments.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Reaction Examples Experiments Epsom salt and water create an interesting endothermic chemical reaction. You can use these when asked to cite an example or to get ideas to set up a demonstration of an. The reaction between vinegar and bicarbonate soda can be written as: Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as. Endothermic Reaction Examples Experiments.
From ar.inspiredpencil.com
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Experiments This is an endothermic reaction, which. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Examples of endothermic processes include the melting of ice and the depressurization of. Endothermic Reaction Examples Experiments.
From www.vedantu.com
Explain Endothermic Reaction with examples? Endothermic Reaction Examples Experiments Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. The reaction between vinegar and bicarbonate soda can be written as: Vinegar + bicarbonate. Endothermic Reaction Examples Experiments.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give Endothermic Reaction Examples Experiments You can use these when asked to cite an example or to get ideas to set up a demonstration of an. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. Epsom salt and water create an interesting endothermic chemical reaction. This is what makes it a fun demonstration. Endothermic Reaction Examples Experiments.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Examples Experiments Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). You can use these when asked to cite an example or to get ideas to set up a demonstration of an. Here's a list. Endothermic Reaction Examples Experiments.
From animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Experiments In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. Epsom salt and water create an interesting endothermic chemical reaction. Vinegar + bicarbonate soda —> carbon dioxide + water. Endothermic Reaction Examples Experiments.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Reaction Examples Experiments You can use these when asked to cite an example or to get ideas to set up a demonstration of an. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions. Endothermic Reaction Examples Experiments.
From fatimakruwgamble.blogspot.com
Examples of Endothermic and Exothermic Reactions FatimakruwGamble Endothermic Reaction Examples Experiments You can use these when asked to cite an example or to get ideas to set up a demonstration of an. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy,. Endothermic Reaction Examples Experiments.
From www.dreamstime.com
Exothermic and Endothermic Reactions in Chemistry Stock Illustration Endothermic Reaction Examples Experiments Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. Epsom salt and water create an interesting endothermic chemical reaction. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). You can use these when. Endothermic Reaction Examples Experiments.
From www.fizzicseducation.com.au
Endothermic reaction Fizzics Education Endothermic Reaction Examples Experiments This is an endothermic reaction, which. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). Here's a list of examples of endothermic reactions. You can use. Endothermic Reaction Examples Experiments.
From animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Experiments In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. You can use these when asked to cite an example or to get ideas to set up a demonstration of an. This is an endothermic reaction, which. Epsom salt and water create an interesting endothermic. Endothermic Reaction Examples Experiments.
From mavink.com
Exothermic Reaction Class 10 Endothermic Reaction Examples Experiments This is an endothermic reaction, which. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. The reaction between vinegar and bicarbonate soda can be written as: Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. An. Endothermic Reaction Examples Experiments.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Examples Experiments Here's a list of examples of endothermic reactions. Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. Examples of endothermic reactions. Endothermic Reaction Examples Experiments.
From www.pinterest.com
Endothermic Reactions Chemistry classroom, Teaching science, Teaching Endothermic Reaction Examples Experiments Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Here's a list of examples of endothermic reactions. This is what makes. Endothermic Reaction Examples Experiments.
From studyzoneharper.z21.web.core.windows.net
Endothermic Vs Exothermic Practice Problems Endothermic Reaction Examples Experiments This is what makes it a fun demonstration and effective teaching activity. Epsom salt and water create an interesting endothermic chemical reaction. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. You can use these when asked to cite an example or to get ideas to set up a demonstration of an. An endothermic reaction is a. Endothermic Reaction Examples Experiments.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Experiments This is what makes it a fun demonstration and effective teaching activity. In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. Examples of. Endothermic Reaction Examples Experiments.
From evulpo.com
Exothermic and endothermic reactions and catalysts Science Endothermic Reaction Examples Experiments Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. This is what makes it a. Endothermic Reaction Examples Experiments.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Examples Experiments Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. Epsom salt and water create an interesting endothermic chemical reaction. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). This is an endothermic reaction,. Endothermic Reaction Examples Experiments.
From in.pinterest.com
Learn about exothermic and endothermic reactions in this blog post here Endothermic Reaction Examples Experiments Epsom salt and water create an interesting endothermic chemical reaction. An endothermic reaction is a chemical reaction that absorbs. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. You can use these when asked to cite an example or to get ideas to set up a demonstration of an. Examples of endothermic processes include the melting of. Endothermic Reaction Examples Experiments.
From www.youtube.com
simple endothermic reaction YouTube Endothermic Reaction Examples Experiments An endothermic reaction is a chemical reaction that absorbs. The reaction between vinegar and bicarbonate soda can be written as: Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. This is what makes it. Endothermic Reaction Examples Experiments.
From www.aiophotoz.com
What Is Endothermic Reaction Images and Photos finder Endothermic Reaction Examples Experiments This is an endothermic reaction, which. In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. This is what makes it a fun demonstration and effective teaching activity. Here's a list of examples of endothermic reactions. An endothermic reaction is a chemical reaction that absorbs.. Endothermic Reaction Examples Experiments.
From www.pinterest.com
For this Endothermic and Exothermic Reactions Lab, students will Endothermic Reaction Examples Experiments Epsom salt and water create an interesting endothermic chemical reaction. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). Here's a list of examples of endothermic. Endothermic Reaction Examples Experiments.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Examples Experiments Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. This is an endothermic reaction, which. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Here's a list of examples of endothermic reactions. An endothermic reaction is a chemical reaction that absorbs. The reaction. Endothermic Reaction Examples Experiments.
From treatybottle13.pythonanywhere.com
Stunning Example Of Exothermic Reaction Class 10 Ib Math Hl Formula Sheet Endothermic Reaction Examples Experiments This is an endothermic reaction, which. Epsom salt and water create an interesting endothermic chemical reaction. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). Examples of. Endothermic Reaction Examples Experiments.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Experiments Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. You can use these when asked to cite an example or to get ideas to set up a demonstration of an. Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. An endothermic process or reaction absorbs energy in the form of. Endothermic Reaction Examples Experiments.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Reaction Examples Experiments This is what makes it a fun demonstration and effective teaching activity. In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. This is an endothermic reaction, which. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. Examples. Endothermic Reaction Examples Experiments.
From www.pinterest.it
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Reaction Examples Experiments Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. This is what makes it a fun demonstration and effective teaching activity. Examples of endothermic processes include the. Endothermic Reaction Examples Experiments.
From signalticket9.pythonanywhere.com
Simple Endothermic Reactions With Equations Reaction Balanced Equation Endothermic Reaction Examples Experiments In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Epsom salt and water create an interesting endothermic chemical reaction. In this simple chemistry activity, your student will be able to feel the results of the chemical reaction, as well as to measure what happened. Students measure the temperature changes. Endothermic Reaction Examples Experiments.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Reaction Examples Experiments You can use these when asked to cite an example or to get ideas to set up a demonstration of an. Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. Here's a list of examples of endothermic reactions. Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying. Endothermic Reaction Examples Experiments.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Experiments Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. The reaction between vinegar and bicarbonate soda can be written as: Here's a list of examples of endothermic reactions. In this simple chemistry. Endothermic Reaction Examples Experiments.
From proper-cooking.info
Endothermic And Exothermic Reaction Examples Endothermic Reaction Examples Experiments Here's a list of examples of endothermic reactions. Epsom salt and water create an interesting endothermic chemical reaction. Vinegar + bicarbonate soda —> carbon dioxide + water + sodium acetate. The experiments can also be used to revise different types of chemical reaction and, with some classes, chemical formulae and equations. The reaction between vinegar and bicarbonate soda can be. Endothermic Reaction Examples Experiments.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Examples Experiments Here's a list of examples of endothermic reactions. An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). Students measure the temperature changes in different reactions taking place in a polystyrene cup, classifying the reactions as exothermic or endothermic. This is what makes it a fun demonstration and. Endothermic Reaction Examples Experiments.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Reaction Examples Experiments An endothermic process or reaction absorbs energy in the form of heat (endergonic processes or reactions absorb energy, not necessarily as heat). The reaction between vinegar and bicarbonate soda can be written as: In this demonstration or class experiment, students observe an endothermic reaction between solid hydrated barium hydroxide and solid ammonium chloride. Epsom salt and water create an interesting. Endothermic Reaction Examples Experiments.
From improove.in
Experiment 16 Heat generation during reaction Improove Endothermic Reaction Examples Experiments Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The reaction between vinegar and bicarbonate soda can be written as: Examples of endothermic processes include the melting of ice and the depressurization of a pressurized can. This is an endothermic reaction, which. An endothermic reaction is a chemical reaction that absorbs. The experiments can also. Endothermic Reaction Examples Experiments.