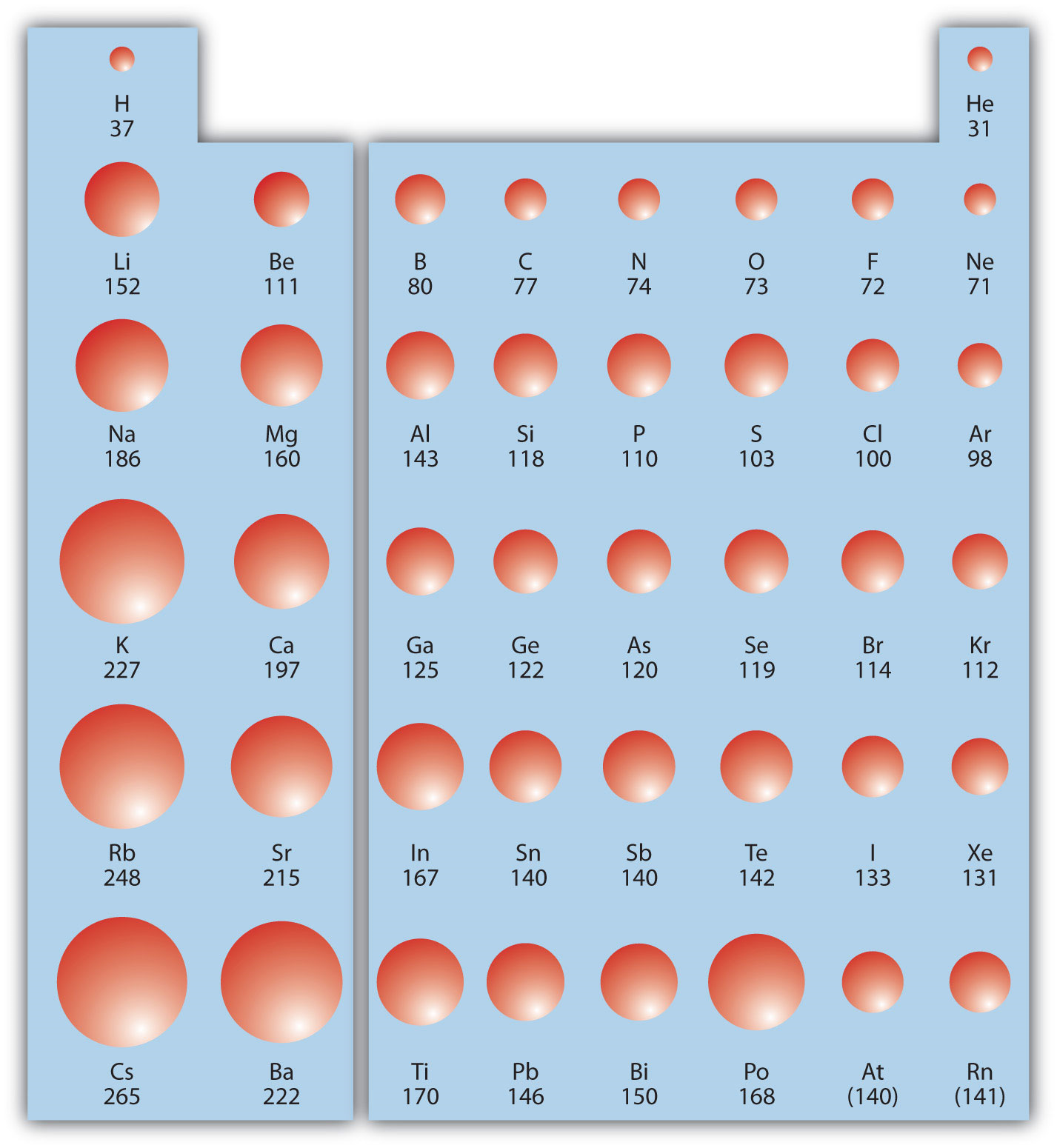
Smallest Atom In Group 14 . That element is carbon, c. This is due to two important qualities of carbon: General electronic configuration of carbon family is. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. Its small size and its unique electron configuration. The element at the very top of group 14 will have the smallest atoms. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Compare the molecular structures of co 2 and sio 2 below: It is also known as the carbon group. Group 14 elements show the greatest diversity in chemical behavior of any group;
from chem.libretexts.org
It is also known as the carbon group. General electronic configuration of carbon family is. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. This is due to two important qualities of carbon: Its small size and its unique electron configuration. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). The element at the very top of group 14 will have the smallest atoms. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. Group 14 elements show the greatest diversity in chemical behavior of any group;
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic
Smallest Atom In Group 14 Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Compare the molecular structures of co 2 and sio 2 below: The element at the very top of group 14 will have the smallest atoms. Group 14 elements show the greatest diversity in chemical behavior of any group; Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Its small size and its unique electron configuration. That element is carbon, c. This is due to two important qualities of carbon: General electronic configuration of carbon family is. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. It is also known as the carbon group. The carbon family elements (silicon) central element to electronic technology and artificial intelligences.
From cezajnau.blob.core.windows.net
Weight Of A Periodic Table at Traci Dowell blog Smallest Atom In Group 14 Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). The carbon family elements (silicon) central element to electronic technology and artificial intelligences. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group.. Smallest Atom In Group 14.
From courses.lumenlearning.com
Electrons Biology for Majors I Smallest Atom In Group 14 It is also known as the carbon group. General electronic configuration of carbon family is. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). Group 14 elements show the greatest diversity in chemical behavior of any group; Compare the molecular structures of co 2 and sio 2 below: That element is carbon, c. Its small. Smallest Atom In Group 14.
From www.teachoo.com
Molecules and Compounds Definition, Differenences [in Table Form] Smallest Atom In Group 14 Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). Its small size and its unique electron configuration. Compare the molecular structures of co 2 and sio 2 below: Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. That element is carbon, c. The element at the. Smallest Atom In Group 14.
From www.numerade.com
SOLVEDGive the symbol of the element (a) in group 14 that has the Smallest Atom In Group 14 Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Its small size and its unique electron configuration. This is due to two important qualities of carbon: Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. It is also known as the carbon group. Compare the. Smallest Atom In Group 14.
From www.askiitians.com
Classification of Elements & Periodicity in Properties askIITians Smallest Atom In Group 14 Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. The element at the very top of group 14 will have the smallest atoms. It is also known as the carbon group. Compare the molecular structures of co 2 and sio 2 below: Its small size and its unique electron configuration. The carbon family elements. Smallest Atom In Group 14.
From stock.adobe.com
Table showing electron orbital configuration of the smallest atoms Smallest Atom In Group 14 Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. Group 14 elements show the greatest diversity in chemical behavior of any group; The carbon family elements (silicon) central element to electronic technology and artificial intelligences. The element at the very. Smallest Atom In Group 14.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar Smallest Atom In Group 14 It is also known as the carbon group. General electronic configuration of carbon family is. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. This is due to two important qualities of carbon: The element at the very top of group 14 will have the smallest atoms. Group 14 elements show the greatest diversity. Smallest Atom In Group 14.
From socratic.org
Which are the smallest and largest atoms? Socratic Smallest Atom In Group 14 Its small size and its unique electron configuration. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). This is due to two important qualities of carbon: That element is carbon, c. General electronic configuration of carbon family is. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this. Smallest Atom In Group 14.
From qnewshub.com
World's Smallest Atommemory Unit Created QNewsHub Smallest Atom In Group 14 That element is carbon, c. This is due to two important qualities of carbon: It is also known as the carbon group. Compare the molecular structures of co 2 and sio 2 below: Group 14 elements show the greatest diversity in chemical behavior of any group; The element at the very top of group 14 will have the smallest atoms.. Smallest Atom In Group 14.
From chemassist.blogspot.com
ChemAssist Groups of the Periodic Table Smallest Atom In Group 14 Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Its small size and its unique electron configuration. That element is carbon, c. It is also known as the carbon group. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. This is due to two important. Smallest Atom In Group 14.
From utedzz.blogspot.com
Largest Atomic Radius Periodic Table Periodic Table Timeline Smallest Atom In Group 14 Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. This is due to two important qualities of carbon: Its small size and its unique electron configuration. Compare the molecular structures of co 2 and sio 2 below: Group 14 elements show the greatest diversity in chemical behavior of any group; That element is carbon,. Smallest Atom In Group 14.
From www.chem.fsu.edu
Electron Configurations Smallest Atom In Group 14 Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). Group 14 elements show the greatest diversity in chemical behavior of any group; General electronic configuration of carbon family is. This is due to two important qualities of. Smallest Atom In Group 14.
From www.numerade.com
SOLVEDIndicate the smallest and the largest species (atom or ion) in Smallest Atom In Group 14 This is due to two important qualities of carbon: Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. It is also known as the carbon group. The element at the very top of group 14 will have the. Smallest Atom In Group 14.
From lillynewssullivan.blogspot.com
Which of the Following Atoms Has the Smallest Radius Smallest Atom In Group 14 This is due to two important qualities of carbon: Group 14 elements show the greatest diversity in chemical behavior of any group; Compare the molecular structures of co 2 and sio 2 below: It is also known as the carbon group. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. Its small size and its unique. Smallest Atom In Group 14.
From www.gizmodo.com.au
We've Measured The Smallest Ever Forces Between Atoms Gizmodo Australia Smallest Atom In Group 14 Its small size and its unique electron configuration. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. It is also known as the carbon group. Group 14 elements show the greatest diversity in chemical behavior of any group; That element is carbon, c. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl). Smallest Atom In Group 14.
From www.science-sparks.com
What is an atom? Smallest Atom In Group 14 Its small size and its unique electron configuration. General electronic configuration of carbon family is. The element at the very top of group 14 will have the smallest atoms. Group 14 elements show the greatest diversity in chemical behavior of any group; Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group.. Smallest Atom In Group 14.
From sciencenotes.org
Atomic Radius and Ionic Radius Smallest Atom In Group 14 Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. General electronic configuration of carbon family is. It is also known as the carbon group. Group 14 elements show the greatest diversity in chemical behavior of any group; The carbon family elements (silicon) central element to electronic technology and artificial intelligences. This is due to. Smallest Atom In Group 14.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Smallest Atom In Group 14 Its small size and its unique electron configuration. Compare the molecular structures of co 2 and sio 2 below: That element is carbon, c. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. General electronic configuration of carbon family is. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are. Smallest Atom In Group 14.
From slideplayer.com
© 2015 Pearson Education, Inc. ppt download Smallest Atom In Group 14 This is due to two important qualities of carbon: The element at the very top of group 14 will have the smallest atoms. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. Group 14 elements show the greatest diversity in chemical behavior of any group; Covalent bond strengths decease with increasing atomic size, and ionization energies. Smallest Atom In Group 14.
From mungfali.com
Chemistry Atom Structure Smallest Atom In Group 14 The carbon family elements (silicon) central element to electronic technology and artificial intelligences. That element is carbon, c. Group 14 elements show the greatest diversity in chemical behavior of any group; Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Compare the molecular structures of co 2 and sio 2 below:. Smallest Atom In Group 14.
From theawesomer.com
How Small Is An Atom? Smallest Atom In Group 14 This is due to two important qualities of carbon: Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). The element at the very top of group 14 will have the smallest atoms. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. Compare the molecular structures of co 2 and sio 2. Smallest Atom In Group 14.
From courses.lumenlearning.com
Molecular and Ionic Compounds Chemistry I Smallest Atom In Group 14 Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. It is also known as the carbon group. This is due to two important qualities of carbon: Compare the molecular structures of co 2 and sio 2 below: Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this. Smallest Atom In Group 14.
From wiredatalizheyaojn.z22.web.core.windows.net
Bohr Model Of The First 20 Elements Smallest Atom In Group 14 Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. General electronic configuration of carbon family is. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. This is due to two important qualities. Smallest Atom In Group 14.
From www.slideserve.com
PPT Atom = smallest unit of an element Molecule = smallest unit of Smallest Atom In Group 14 Its small size and its unique electron configuration. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). It is also known as the carbon group. That element is carbon, c. This is due to two important qualities. Smallest Atom In Group 14.
From www.doubtnut.com
Which elements have the largest and the smallest atoms? Smallest Atom In Group 14 General electronic configuration of carbon family is. The element at the very top of group 14 will have the smallest atoms. This is due to two important qualities of carbon: Its small size and its unique electron configuration. Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). It is also known as the carbon group.. Smallest Atom In Group 14.
From socratic.org
For a given Period, which is the smallest atom? Socratic Smallest Atom In Group 14 Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. General electronic configuration of carbon family is. It is also known as the carbon group. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this. Smallest Atom In Group 14.
From www.nagwa.com
Question Video Determining Which Alkali Metal Has the Smallest Atomic Smallest Atom In Group 14 The element at the very top of group 14 will have the smallest atoms. This is due to two important qualities of carbon: Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. Group 14 elements show the greatest. Smallest Atom In Group 14.
From www.ck12.org
Periodic Trends in Atomic Size CK12 Foundation Smallest Atom In Group 14 General electronic configuration of carbon family is. That element is carbon, c. Group 14 elements show the greatest diversity in chemical behavior of any group; Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. The element at the very top of group 14 will have the smallest atoms. Compare the molecular. Smallest Atom In Group 14.
From klayuppfq.blob.core.windows.net
Why Is Ca Bigger Than Ca2 at Doreen Triana blog Smallest Atom In Group 14 Its small size and its unique electron configuration. The carbon family elements (silicon) central element to electronic technology and artificial intelligences. That element is carbon, c. It is also known as the carbon group. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. General electronic configuration of carbon family is. The element at the. Smallest Atom In Group 14.
From www.chegg.com
Solved Use the information found in the periodic table below Smallest Atom In Group 14 It is also known as the carbon group. The element at the very top of group 14 will have the smallest atoms. Its small size and its unique electron configuration. General electronic configuration of carbon family is. That element is carbon, c. Compare the molecular structures of co 2 and sio 2 below: Group 14 includes carbon (c), silicon (si),. Smallest Atom In Group 14.
From www.youtube.com
What is the Smallest Atom in the World? Class 10 Physics. CBSE 10th Smallest Atom In Group 14 Compare the molecular structures of co 2 and sio 2 below: Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. Group 14 elements show the greatest diversity in chemical behavior of any group; General electronic configuration of carbon family is. It is also known as the carbon group. The carbon family elements (silicon) central. Smallest Atom In Group 14.
From www.slideserve.com
PPT Anatomy & Physiology Basic Chemistry Chapter 2 PowerPoint Smallest Atom In Group 14 Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Compare the molecular structures of co 2 and sio 2 below: This is due to two important qualities of carbon: That element is carbon, c. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. Group 14. Smallest Atom In Group 14.
From abcinfolinks.blogspot.com
abc blog Discovery of an ATOM. Smallest Atom In Group 14 Compare the molecular structures of co 2 and sio 2 below: General electronic configuration of carbon family is. This is due to two important qualities of carbon: Group 14 includes carbon (c), silicon (si), germanium (ge), tin (sn) and lead (pb). It is also known as the carbon group. That element is carbon, c. Group 14 elements show the greatest. Smallest Atom In Group 14.
From socratic.org
How to arrange the following atoms and ions in order of increasing Smallest Atom In Group 14 Its small size and its unique electron configuration. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. Carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl) are members of this group. Compare the molecular structures of co 2 and sio 2 below: That element is carbon, c. It is also. Smallest Atom In Group 14.
From manetdesktop.weebly.com
Atoms are the smallest particles of matter Smallest Atom In Group 14 Compare the molecular structures of co 2 and sio 2 below: Its small size and its unique electron configuration. Covalent bond strengths decease with increasing atomic size, and ionization energies are greater than expected,. It is also known as the carbon group. Group 14 elements show the greatest diversity in chemical behavior of any group; The element at the very. Smallest Atom In Group 14.