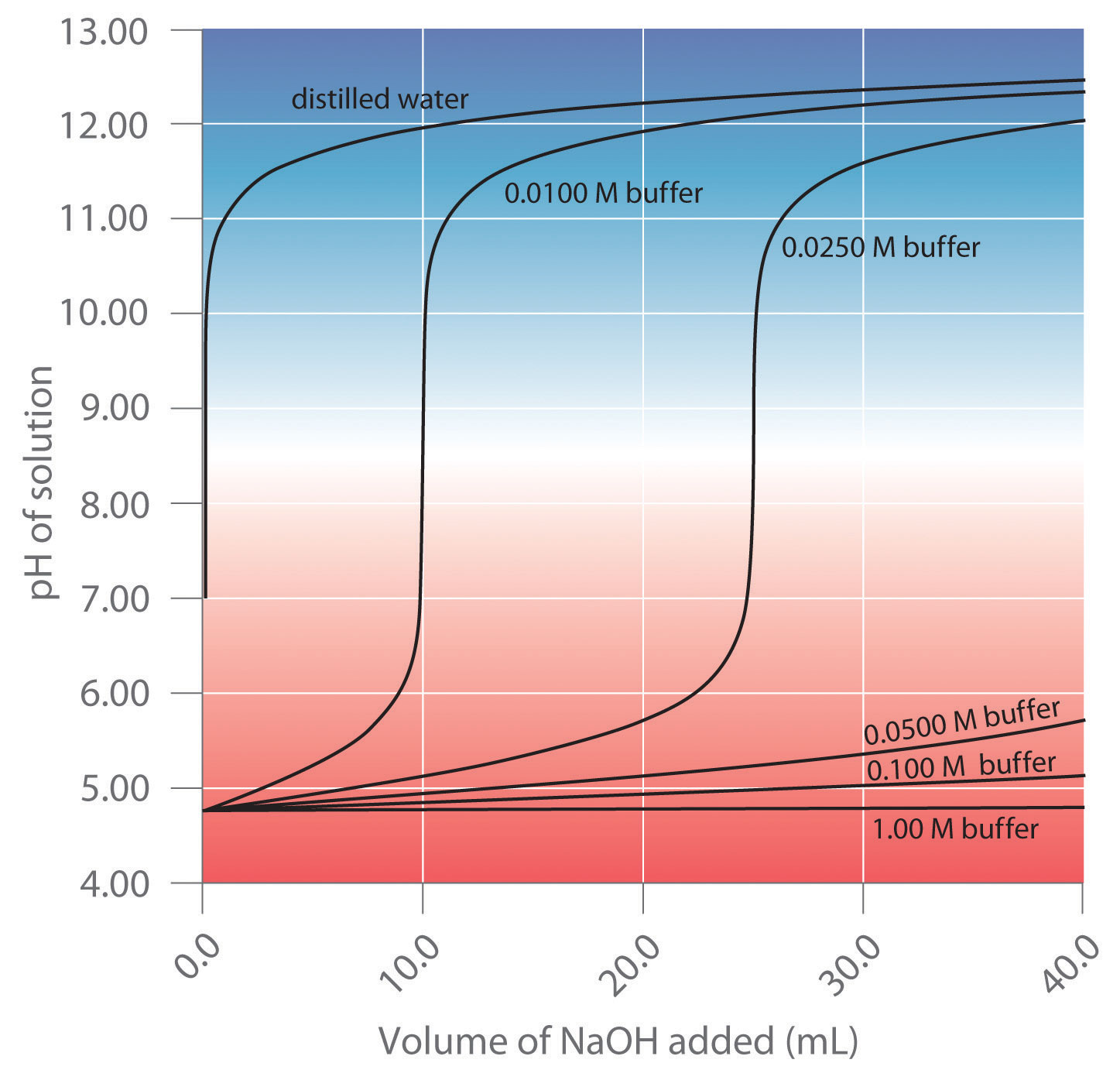
What Is A Buffer Range . A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small amounts of added acid or base, thus. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. An important property of buffer solutions is.
from saylordotorg.github.io
An important property of buffer solutions is. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. It is able to neutralize small amounts of added acid or base, thus. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes.
Buffers
What Is A Buffer Range An important property of buffer solutions is. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. An important property of buffer solutions is. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
From www.youtube.com
Buffer range example YouTube What Is A Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. This characteristic makes buffers important in biological and chemical applications where ph stability is. What Is A Buffer Range.
From studylib.net
Buffer Capacity What Is A Buffer Range A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. An. What Is A Buffer Range.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital What Is A Buffer Range Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. An important property of buffer solutions is. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. This characteristic makes buffers important in biological and chemical. What Is A Buffer Range.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Is A Buffer Range This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize small amounts of added acid or base, thus. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is an aqueous solution used to keep the ph of a. What Is A Buffer Range.
From ruslan179144.blogspot.com
Define Definition Of Buffer Capacity Robert Romero Coiffure What Is A Buffer Range If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. The. What Is A Buffer Range.
From www.researchgate.net
Any suggestion of buffer solution for photocatalysis using ZnO What Is A Buffer Range Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.. What Is A Buffer Range.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Is A Buffer Range A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its. What Is A Buffer Range.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration What Is A Buffer Range An important property of buffer solutions is. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer consists of a weak acid and. What Is A Buffer Range.
From www.youtube.com
Buffers and pH Biology YouTube What Is A Buffer Range Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer consists of a weak acid and its conjugate base or a weak. What Is A Buffer Range.
From labpedia.net
Acidbase Balance Part 3 Respiratory Acidosis and Respiratory What Is A Buffer Range Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial.. What Is A Buffer Range.
From dxouambuf.blob.core.windows.net
How To Use A Buffer at Albert Hill blog What Is A Buffer Range It is able to neutralize small amounts of added acid or base, thus. An important property of buffer solutions is. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate. What Is A Buffer Range.
From saylordotorg.github.io
Buffers What Is A Buffer Range If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers. What Is A Buffer Range.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Is A Buffer Range Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions are aqueous solutions, consisting of a. What Is A Buffer Range.
From www.wizeprep.com
Buffer Range Problems Wize University Chemistry Textbook Wizeprep What Is A Buffer Range A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. An important property of buffer solutions is. A buffer is a solution that can resist ph change upon the addition of an acidic. What Is A Buffer Range.
From www.youtube.com
NCEA L3 Chem Buffers, Buffer Region & Preparation of Buffer Solutions What Is A Buffer Range If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. This. What Is A Buffer Range.
From www.crawfordscientific.com
Buffer choice for HPLC separations What Is A Buffer Range An important property of buffer solutions is. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it.. What Is A Buffer Range.
From dxobuticl.blob.core.windows.net
Commonly Used Buffers In The Laboratory at Savannah Osgood blog What Is A Buffer Range A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. An important property of buffer solutions is. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. A buffer consists of a weak acid and its conjugate base or a weak base. What Is A Buffer Range.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Is A Buffer Range It is able to neutralize small amounts of added acid or base, thus. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffer capacity is the amount of acid. What Is A Buffer Range.
From www.slideserve.com
PPT Chapter Two Water The Solvent for Biochemical Reactions What Is A Buffer Range The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. An important property of buffer solutions is. Buffer solutions are aqueous solutions,. What Is A Buffer Range.
From www.youtube.com
Buffers and Titration Curves YouTube What Is A Buffer Range A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. A buffer is. What Is A Buffer Range.
From www.slideserve.com
PPT Chapter 14 Equilibria in AcidBase Solutions PowerPoint What Is A Buffer Range An important property of buffer solutions is. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. A buffer is a solution that can resist ph change upon the addition of an. What Is A Buffer Range.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Is A Buffer Range A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. An important property of buffer solutions is. This characteristic makes buffers important in biological and chemical. What Is A Buffer Range.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is A Buffer Range A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base. What Is A Buffer Range.
From www.youtube.com
Buffer Range and Buffer Capacity.mp4 YouTube What Is A Buffer Range This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize small amounts of added acid or base, thus. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers are characterized by the ph range over which they can maintain a. What Is A Buffer Range.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies What Is A Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. This characteristic makes buffers important in biological and chemical applications where ph stability is. What Is A Buffer Range.
From www.youtube.com
An Introduction to Buffers and Their Ranges YouTube What Is A Buffer Range A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffer capacity. What Is A Buffer Range.
From studylib.net
Good's buffers (biological buffers) What Is A Buffer Range A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. A buffer is a solution. What Is A Buffer Range.
From saylordotorg.github.io
Buffers What Is A Buffer Range If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. This characteristic makes buffers important in biological and chemical applications where ph stability. What Is A Buffer Range.
From www.semanticscholar.org
Table 1 from Buffer solutions in drug formulation and processing How What Is A Buffer Range A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. Buffer capacity is the amount of acid or base that can be added before the. What Is A Buffer Range.
From www.youtube.com
Buffer range and capacity YouTube What Is A Buffer Range Buffer capacity is the amount of acid or base that can be added before the ph of a buffer changes. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or. What Is A Buffer Range.
From slideplayer.com
Buffer Effectiveness, Titrations, and pH Curves ppt download What Is A Buffer Range An important property of buffer solutions is. The buffer capacity is a measure of resistant a particular solution is resistant to change in ph when an acid or a base is added to it. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. This characteristic makes buffers important in biological. What Is A Buffer Range.
From www.chegg.com
Solved Consider the following questions about buffer What Is A Buffer Range A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate base or a. What Is A Buffer Range.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Is A Buffer Range Buffers are characterized by the ph range over which they can maintain a more or less constant ph and by their buffer capacity, the amount of. A buffer is an aqueous solution used to keep the ph of a solution nearly constant. An important property of buffer solutions is. It is able to neutralize small amounts of added acid or. What Is A Buffer Range.
From www.youtube.com
8 Buffer Calculations 3 YouTube What Is A Buffer Range A buffer is an aqueous solution used to keep the ph of a solution nearly constant. This characteristic makes buffers important in biological and chemical applications where ph stability is crucial. It is able to neutralize small amounts of added acid or base, thus. Buffer solutions are aqueous solutions, consisting of a mixture of a weak acid and its conjugate. What Is A Buffer Range.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint What Is A Buffer Range An important property of buffer solutions is. It is able to neutralize small amounts of added acid or base, thus. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid. If you remember high school chemistry or took a college course like chemistry 101, you will have conducted a titration test.. What Is A Buffer Range.