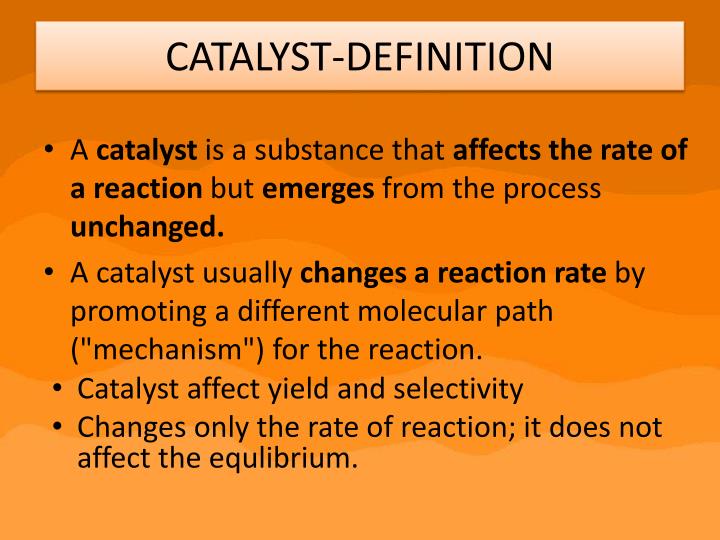
Catalytic Effect Meaning . In general, catalytic action is a chemical reaction between the catalyst and a reactant. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. By the end of this section, you will be able to: Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Explain the function of a catalyst in terms of reaction mechanisms and. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
from www.slideserve.com
A very small amount of catalyst is required to alter the reaction rate. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. By the end of this section, you will be able to: If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Explain the function of a catalyst in terms of reaction mechanisms and. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint
Catalytic Effect Meaning Explain the function of a catalyst in terms of reaction mechanisms and. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. Explain the function of a catalyst in terms of reaction mechanisms and. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. A very small amount of catalyst is required to alter the reaction rate. By the end of this section, you will be able to: In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction.
From www.slideserve.com
PPT §10.5 Catalytic reaction PowerPoint Presentation, free download Catalytic Effect Meaning In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. A very small amount of catalyst is required to alter the reaction rate. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Explain the function of a catalyst in terms of reaction mechanisms and. If you. Catalytic Effect Meaning.
From www.studocu.com
Catalytic efficiency How enzymes work Catalytic efficiency = kcat Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. By the end of this section, you will be able to: If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. Enzymes are naturally occurring catalysts responsible for many essential. Catalytic Effect Meaning.
From www.chemistrystudent.com
Catalysts (ALevel) ChemistryStudent Catalytic Effect Meaning In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Explain the function of a catalyst in terms of reaction mechanisms and. Catalyst,. Catalytic Effect Meaning.
From www.researchgate.net
Putative mechanism of the catalytic effect of two peroxybicarbonate Catalytic Effect Meaning If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. A very small amount of catalyst is required to alter the reaction rate. In chemistry, a catalytic substance or a substance with. Catalytic Effect Meaning.
From www.researchgate.net
Entropydriven DNA catalysis reactions. (A) Components of the catalytic Catalytic Effect Meaning In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. Explain the function of a catalyst in terms of reaction mechanisms. Catalytic Effect Meaning.
From 2012books.lardbucket.org
Catalysis Catalytic Effect Meaning Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Explain the function of a catalyst in terms of reaction mechanisms and. By the end of this section, you will be able to:. Catalytic Effect Meaning.
From study.com
Effect of Catalysts on Rates of Reaction Lesson Catalytic Effect Meaning In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the. Catalytic Effect Meaning.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint Catalytic Effect Meaning If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. By the end of this section, you will be able to: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing. Catalytic Effect Meaning.
From www.researchgate.net
Catalytic effects of template variants. The optimized sequence is shown Catalytic Effect Meaning By the end of this section, you will be able to: In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. Explain the function of a catalyst in terms of reaction mechanisms and. A very small amount of catalyst is required to alter the reaction rate. Catalyst, in. Catalytic Effect Meaning.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. Explain the function of a catalyst in terms of. Catalytic Effect Meaning.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalytic Effect Meaning In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. By the end of this section, you will be able to: If you describe a person or thing as having a catalytic. Catalytic Effect Meaning.
From www.catalystseurope.org
What are catalysts? Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Explain the function of a catalyst in terms of reaction mechanisms and. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysis is a process of. Catalytic Effect Meaning.
From eng.nju.edu.cn
Binuclear Cu complex catalysis enabling Li Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. In general, catalytic action is a chemical reaction between the catalyst and a reactant. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. Explain the function of a catalyst in terms of reaction mechanisms. Catalytic Effect Meaning.
From www.researchgate.net
The relationship between ligands and catalytic effect. The catalytic Catalytic Effect Meaning Explain the function of a catalyst in terms of reaction mechanisms and. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. A very small amount of catalyst is required to alter the reaction rate. By the end of this section, you will be able to: In. Catalytic Effect Meaning.
From www.researchgate.net
Effect of the catalytic efficiency Download Scientific Diagram Catalytic Effect Meaning Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a. Catalytic Effect Meaning.
From www.youtube.com
6.2.6 / 6.2.7 Describe the effect of a catalyst on a chemical reaction Catalytic Effect Meaning Explain the function of a catalyst in terms of reaction mechanisms and. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. By the end of this section, you will be able to: If you describe a person or thing as having a. Catalytic Effect Meaning.
From www.slideserve.com
PPT Biochemistry PowerPoint Presentation, free download ID5701075 Catalytic Effect Meaning Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known. Catalytic Effect Meaning.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation Catalytic Effect Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. By the end of this section, you will be able to: A very small amount of catalyst is required to alter the reaction rate. In general, catalytic action is a chemical reaction between the catalyst and a reactant. In chemistry, a catalytic substance or. Catalytic Effect Meaning.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. Explain the function of a catalyst in terms of reaction mechanisms and. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. By the end of this section, you will be able to: If you describe a person or thing as having a catalytic effect, you. Catalytic Effect Meaning.
From sop4cv.com
Catalysis Catalytic Effect Meaning Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. Catalytic Effect Meaning.
From www.britannica.com
Catalysis Chemistry, Classification, & Chemical Reactions Britannica Catalytic Effect Meaning Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. If you describe a person or thing as having a catalytic. Catalytic Effect Meaning.
From www.researchgate.net
3 Catalytic effect of different metals on oxidation rate. Download Catalytic Effect Meaning By the end of this section, you will be able to: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is. Catalytic Effect Meaning.
From mavink.com
Catalytic Cracking Diagram Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. By the end of this section, you will be able to: Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. If you describe a person or thing as having a catalytic effect, you. Catalytic Effect Meaning.
From www.mdpi.com
Catalysts Special Issue Ionic Liquids in Catalysis Catalytic Effect Meaning Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as a catalyst. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at. Catalytic Effect Meaning.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst Catalytic Effect Meaning If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. By the end of this section, you will be able to: In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction.. Catalytic Effect Meaning.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID Catalytic Effect Meaning In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A very small amount of catalyst is required to alter the reaction rate. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance. Catalytic Effect Meaning.
From bianca-well-hooper.blogspot.com
Example of Combination Reaction in Which Catalyst Is Used Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. By the end of this section, you will be able to: Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Catalysis is a process of increasing the rate of a chemical. Catalytic Effect Meaning.
From www.rankred.com
What Is A Catalyst? Definition Types Examples RankRed Catalytic Effect Meaning In general, catalytic action is a chemical reaction between the catalyst and a reactant. By the end of this section, you will be able to: If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. Catalysis is a process of increasing the rate. Catalytic Effect Meaning.
From www.slideserve.com
PPT Enzymes as Biological Catalysts PowerPoint Presentation, free Catalytic Effect Meaning In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Explain the function of a catalyst in terms of reaction mechanisms and. A. Catalytic Effect Meaning.
From thecontentauthority.com
How To Use "Catalytic Effect" In A Sentence Diving Deeper Catalytic Effect Meaning A very small amount of catalyst is required to alter the reaction rate. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Explain the function of a catalyst in terms of reaction mechanisms and. By the end of this section, you will be able to: In general, catalytic action is a chemical reaction between the catalyst and a. Catalytic Effect Meaning.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 Catalytic Effect Meaning Explain the function of a catalyst in terms of reaction mechanisms and. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In general, catalytic action is a chemical reaction between the catalyst and a reactant. If you describe a person or thing as having a catalytic effect, you mean that they cause things. Catalytic Effect Meaning.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Catalytic Effect Meaning Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In chemistry, a catalytic substance or a substance with catalytic properties is a substance which increases the speed of a chemical reaction. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they. Catalytic Effect Meaning.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalytic Effect Meaning If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. Explain the function of a catalyst in terms of reaction mechanisms and. Catalysis is a process of increasing the rate of a chemical reaction by adding a chemical substance which is known as. Catalytic Effect Meaning.
From www.youtube.com
Catalytic Meaning YouTube Catalytic Effect Meaning Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. If you describe a person or thing as having a catalytic effect, you mean that they cause things to happen or they increase the speed at which. By the end of this section, you will be able to: In general, catalytic action is a chemical reaction between the catalyst. Catalytic Effect Meaning.
From www.researchgate.net
Catalytic effect of TM lattice (Ni/Mn/Co) toward side reactions that Catalytic Effect Meaning Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. Explain the function of a catalyst in terms of reaction mechanisms and. A very small amount of catalyst is required to alter the reaction rate. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. If you describe a person or thing as. Catalytic Effect Meaning.