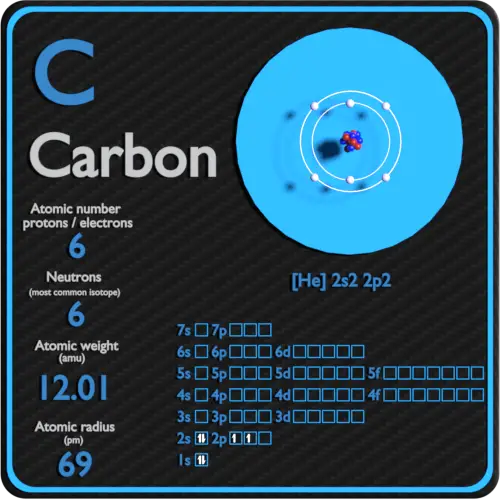
How Many Neutrons Are There In Carbon . Oxygen and carbon are both elements. For example, a carbon atom weighs less than 2. The chemical symbol for carbon is c. Neutron number and mass number of carbon. Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. Mass numbers of typical isotopes of carbon are. It has an atomic weight of 12.011 and a mass number. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of 6. But what makes oxygen different from carbon?
from material-properties.org
Mass numbers of typical isotopes of carbon are. But what makes oxygen different from carbon? Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of 6. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2. The chemical symbol for carbon is c. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Neutron number and mass number of carbon. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth.
Carbone Protons Neutrons Électrons Configuration électronique
How Many Neutrons Are There In Carbon Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. But what makes oxygen different from carbon? Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. It has an atomic weight of 12.011 and a mass number. For example, a carbon atom weighs less than 2. Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of 6. Mass numbers of typical isotopes of carbon are. Neutron number and mass number of carbon. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. The chemical symbol for carbon is c. Oxygen and carbon are both elements.
From material-properties.org
Carbone Protons Neutrons Électrons Configuration électronique How Many Neutrons Are There In Carbon Neutron number and mass number of carbon. Oxygen and carbon are both elements. But what makes oxygen different from carbon? Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. It has an atomic weight of 12.011 and a mass number. For example, a carbon atom weighs less than 2. Mass numbers of typical isotopes of carbon are. Both. How Many Neutrons Are There In Carbon.
From lertnewslozno.blogspot.com
lertNewsLozno How Many Neutrons Are There In Carbon Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Mass numbers of typical isotopes of carbon are. It has an atomic weight of 12.011 and a mass number. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Oxygen and carbon. How Many Neutrons Are There In Carbon.
From www.slideserve.com
PPT Physical Science PowerPoint Presentation, free download ID1589136 How Many Neutrons Are There In Carbon The chemical symbol for carbon is c. Oxygen and carbon are both elements. Neutron number and mass number of carbon. Mass numbers of typical isotopes of carbon are. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. It has an atomic weight of 12.011 and a mass number.. How Many Neutrons Are There In Carbon.
From structureofanatom.weebly.com
About Atomic Structure How Many Neutrons Are There In Carbon Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Neutron number and mass number of carbon. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. It has an atomic weight of 12.011 and a mass number. But what makes oxygen different from carbon? Atoms—and. How Many Neutrons Are There In Carbon.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID5480927 How Many Neutrons Are There In Carbon Oxygen and carbon are both elements. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2. Both are made up of atoms which in turn are made up of protons, neutrons and electrons.. How Many Neutrons Are There In Carbon.
From sitn.hms.harvard.edu
Unexpected Lessons Learned from MidCentury Atomic Bomb Explosions How Many Neutrons Are There In Carbon Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of 6. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. Mass numbers of typical isotopes of carbon are. But what makes oxygen different from carbon? Neutron number and mass number of carbon. Atoms—and. How Many Neutrons Are There In Carbon.
From slideplayer.com
Unit 2 Review. ppt download How Many Neutrons Are There In Carbon However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Neutron number and mass number of carbon. For example, a carbon atom weighs less than 2. But what makes oxygen different from carbon? It has an atomic weight of 12.011 and a mass number. Mass numbers of typical isotopes. How Many Neutrons Are There In Carbon.
From montessorimuddle.org
Drawing Atoms Montessori Muddle How Many Neutrons Are There In Carbon As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. For example, a carbon atom weighs less than 2. Neutron number and mass number of carbon. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain. How Many Neutrons Are There In Carbon.
From www.nuclear-power.com
Carbon Atomic Number Atomic Mass Density of Carbon nuclear How Many Neutrons Are There In Carbon Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. Neutron number and mass number of carbon. Oxygen and carbon are both elements. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. But what. How Many Neutrons Are There In Carbon.
From www.livescience.com
Carbon Facts about an element that is a key ingredient for life on How Many Neutrons Are There In Carbon Neutron number and mass number of carbon. Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of 6. But what makes oxygen different from carbon? Mass numbers of typical isotopes of carbon are. Oxygen and carbon are both elements. The chemical symbol for carbon is c. It has an atomic weight. How Many Neutrons Are There In Carbon.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID2050882 How Many Neutrons Are There In Carbon For example, a carbon atom weighs less than 2. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. The chemical symbol for carbon is c. But what makes. How Many Neutrons Are There In Carbon.
From www.numerade.com
SOLVEDHow many electrons, protons, and neutrons are there in an atom How Many Neutrons Are There In Carbon Neutron number and mass number of carbon. Mass numbers of typical isotopes of carbon are. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Oxygen and carbon are both elements. Atoms—and. How Many Neutrons Are There In Carbon.
From www.expii.com
Electrons — Structure & Properties Expii How Many Neutrons Are There In Carbon However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. Neutron number and mass number of carbon. Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. The chemical symbol for. How Many Neutrons Are There In Carbon.
From slideplayer.com
Title Atomic Properties ppt download How Many Neutrons Are There In Carbon For example, a carbon atom weighs less than 2. But what makes oxygen different from carbon? Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. Neutron number and mass number of carbon. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. The chemical symbol for carbon. How Many Neutrons Are There In Carbon.
From ascensionglossary.com
Carbon Atom Ascension Glossary How Many Neutrons Are There In Carbon Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of. How Many Neutrons Are There In Carbon.
From sklep.foteks.pl
Plakat Molecular structure of a carbon atom. Electrons, protons, and How Many Neutrons Are There In Carbon However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Neutron number and mass number of carbon. Oxygen and carbon are both elements. It has an atomic weight of 12.011 and a mass number. Mass numbers of typical isotopes of carbon are. Carbon is the 6th element in the. How Many Neutrons Are There In Carbon.
From www.alamy.com
Carbon, atom model of carbon12 with 6 protons, 6 neutrons and 6 How Many Neutrons Are There In Carbon As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. The chemical symbol for carbon is c. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Carbon is the 6th element in the periodic table and has a symbol of c. How Many Neutrons Are There In Carbon.
From hadoma.com
How many protons, neutrons and electrons does carbon have? (2022) How Many Neutrons Are There In Carbon The chemical symbol for carbon is c. Oxygen and carbon are both elements. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. But what makes oxygen different from carbon? Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6,. How Many Neutrons Are There In Carbon.
From www.youtube.com
How many electrons protons and neutrons are there in carbon13? YouTube How Many Neutrons Are There In Carbon Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. It has an atomic weight of 12.011 and a mass number. The chemical symbol for carbon is c. Oxygen and carbon are both elements. Neutron number and mass number of carbon. But what makes oxygen different from carbon? For example, a carbon atom weighs less than 2. Mass numbers. How Many Neutrons Are There In Carbon.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer How Many Neutrons Are There In Carbon However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Mass numbers of typical isotopes of carbon are. For example, a carbon atom weighs less than 2. It has an atomic weight of 12.011 and a mass number. Oxygen and carbon are both elements. Neutron number and mass number. How Many Neutrons Are There In Carbon.
From www.alamy.com
Atomic Structure Of Carbon High Resolution Stock Photography and Images How Many Neutrons Are There In Carbon However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Oxygen and carbon are both elements. Neutron number and mass number of carbon. Mass numbers of typical isotopes of carbon are. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. As. How Many Neutrons Are There In Carbon.
From www.expii.com
Neutrons — Structure & Properties Expii How Many Neutrons Are There In Carbon Oxygen and carbon are both elements. Neutron number and mass number of carbon. Mass numbers of typical isotopes of carbon are. Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. For example, a carbon atom weighs less than 2. But what makes oxygen different from carbon? However, the number of neutrons within an atom of an element is. How Many Neutrons Are There In Carbon.
From www.slideserve.com
PPT 33 Counting Atoms PowerPoint Presentation, free download ID How Many Neutrons Are There In Carbon It has an atomic weight of 12.011 and a mass number. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6. How Many Neutrons Are There In Carbon.
From slideplayer.com
Objectives Identify elements common to all living things Describe how How Many Neutrons Are There In Carbon Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of. How Many Neutrons Are There In Carbon.
From stock.adobe.com
Atomic elements showing the nucleus and shells, numbers of electrons How Many Neutrons Are There In Carbon Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. The chemical symbol for carbon is c. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. But. How Many Neutrons Are There In Carbon.
From www.slideserve.com
PPT 1 st Six Weeks Review PowerPoint Presentation ID5908139 How Many Neutrons Are There In Carbon Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. For example, a carbon atom weighs less than 2. The chemical symbol for carbon is c. Atoms—and the protons,. How Many Neutrons Are There In Carbon.
From www.expii.com
Carbon — Role and Importance to Life Expii How Many Neutrons Are There In Carbon But what makes oxygen different from carbon? Neutron number and mass number of carbon. As one of the environmental isotopes, it makes up about 1.1% of all natural carbon on earth. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Oxygen and carbon are both elements. Mass numbers of typical isotopes of. How Many Neutrons Are There In Carbon.
From www.wikihow.com
How to Find the Number of Neutrons in an Atom 11 Steps How Many Neutrons Are There In Carbon Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of 6. But what makes oxygen different from carbon? It has an atomic weight of 12.011 and a mass number. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Atoms—and the protons, neutrons, and. How Many Neutrons Are There In Carbon.
From www.alamy.com
Carbon atom showing six electrons orbiting six protons and six neutrons How Many Neutrons Are There In Carbon The chemical symbol for carbon is c. Carbon is the 6th element in the periodic table and has a symbol of c and atomic number of 6. Neutron number and mass number of carbon. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also. How Many Neutrons Are There In Carbon.
From slideplayer.com
Earth Materials Minerals ppt download How Many Neutrons Are There In Carbon But what makes oxygen different from carbon? For example, a carbon atom weighs less than 2. It has an atomic weight of 12.011 and a mass number. However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Both are made up of atoms which in turn are made up. How Many Neutrons Are There In Carbon.
From chemistry291.blogspot.com
How Many Neutrons Does Carbon Have?Number of Neutrons in Carbon How Many Neutrons Are There In Carbon However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Oxygen and carbon are both elements. For example, a carbon atom weighs less than 2. Neutron number and mass number of carbon. Atoms—and the protons, neutrons, and electrons that compose them—are extremely small. It has an atomic weight of. How Many Neutrons Are There In Carbon.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Carbon (C How Many Neutrons Are There In Carbon Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. However, the number of neutrons within an atom of an element is not defined by the atomic number of. How Many Neutrons Are There In Carbon.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Carbon14 (14C; radiocarbon) How Many Neutrons Are There In Carbon But what makes oxygen different from carbon? Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6 electrons, in order for the atom to maintain an overall net neutral charge. Mass numbers of typical isotopes of carbon are. Neutron number and mass. How Many Neutrons Are There In Carbon.
From slideplayer.com
ATOMS Standard C2 Students will demonstrate an understanding of atomic How Many Neutrons Are There In Carbon However, the number of neutrons within an atom of an element is not defined by the atomic number of that element. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. But what makes oxygen different from carbon? Oxygen and carbon are both elements. As one of the environmental isotopes, it makes up. How Many Neutrons Are There In Carbon.
From www.numerade.com
SOLVEDHow many electrons, protons, and neutrons are there in an atom How Many Neutrons Are There In Carbon Oxygen and carbon are both elements. For example, a carbon atom weighs less than 2. Both are made up of atoms which in turn are made up of protons, neutrons and electrons. Every atom of carbon, c, that exists in the known universe is defined to contain 6 protons, because its atomic number is 6, and must also contain 6. How Many Neutrons Are There In Carbon.