Standard Hydrogen Electrode Oxidation Potential . the oxygen evolution reaction is crucial for energy conversion but faces challenges in catalyst optimization. The oxidation of water to yield molecular. the standard oxidation potential is much like the standard reduction potential. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a. we computationally evaluated the standard hydrogen electrode (she) potential in aqueous phase and the gibbs. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. The structure of the standard hydrogen electrode is described. normal hydrogen electrode: in this example, the standard reduction potential for zn2+(aq) + 2e− → zn(s) is −0.76 v, which means that the. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. in this reaction, because our standard is defined as contributing exactly 0 v (at standard conditions), e° net will be the. the second pathway to percarbonate formation is a straightforward electrochemical oxidation of bicarbonate. graphical representation of the onset potentials of the her/ hor and oer/orr measured using a standard hydrogen. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. the redox electrode which is used by scientists for reference on all half cell potential reactions and which is the.
from www.vedantu.com
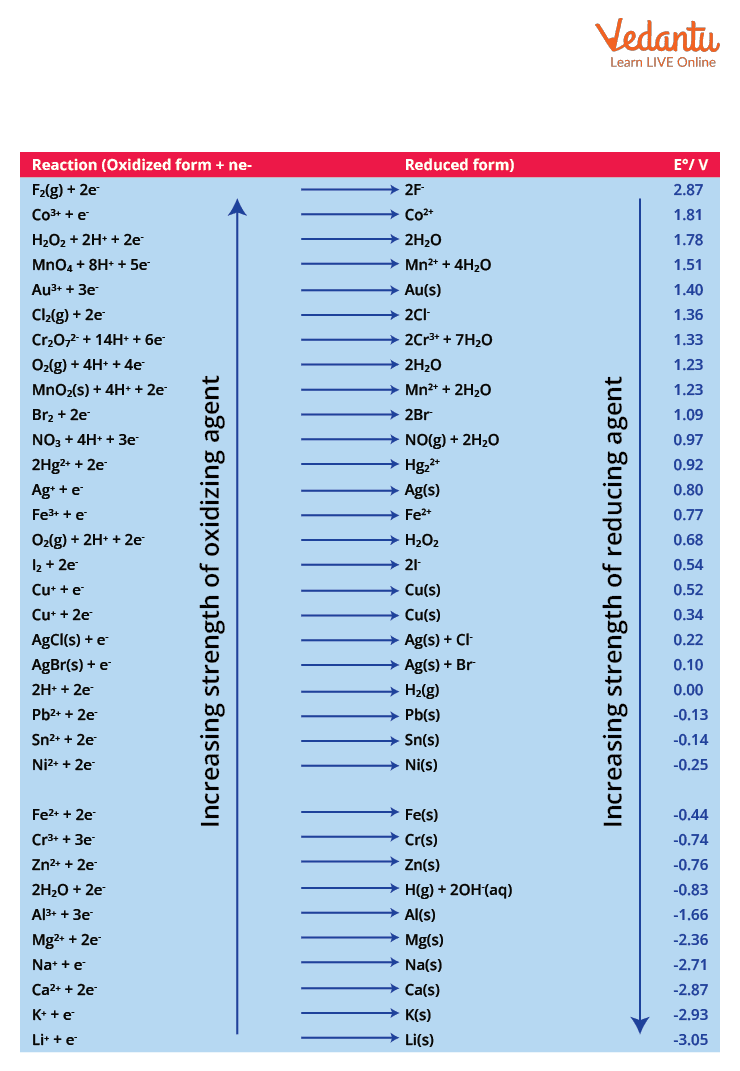
the standard oxidation potential is much like the standard reduction potential. It is the tendency for a species to be oxidized at standard conditions. we computationally evaluated the standard hydrogen electrode (she) potential in aqueous phase and the gibbs. The structure of the standard hydrogen electrode is described. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the oxygen evolution reaction is crucial for energy conversion but faces challenges in catalyst optimization. normal hydrogen electrode: our finding suggests a reactive microenvironment at the interface of the gas diffusion electrode owing to the. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:.
Oxidation Potential and Number Important Concepts and Tips for JEE
Standard Hydrogen Electrode Oxidation Potential the standard oxidation potential is much like the standard reduction potential. the second pathway to percarbonate formation is a straightforward electrochemical oxidation of bicarbonate. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. The oxidation of water to yield molecular. the standard oxidation potential is much like the standard reduction potential. the absolute potential of the standard hydrogen electrode, she, was calculated on the basis of a. It is also written in the form of a half reaction, and an example is shown below. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. to determine an electrode’s potential, a cell is set up using the electrode as one of the electrodes and the second. normal hydrogen electrode: the standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox. The structure of the standard hydrogen electrode is described.
From www.substech.com
standard_electrode_potential.png [SubsTech] Standard Hydrogen Electrode Oxidation Potential graphical representation of the onset potentials of the her/ hor and oer/orr measured using a standard hydrogen. It is the tendency for a species to be oxidized at standard conditions. the redox electrode which is used by scientists for reference on all half cell potential reactions and which is the. normal hydrogen electrode: the standard hydrogen. Standard Hydrogen Electrode Oxidation Potential.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID752723 Standard Hydrogen Electrode Oxidation Potential It is also written in the form of a half reaction, and an example is shown below. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The structure of the standard hydrogen electrode is described. revision notes on 5.3.3 standard electrode & cell potentials for the. Standard Hydrogen Electrode Oxidation Potential.
From www.mdpi.com
Molecules Free FullText Redox Species of Redox Flow Batteries A Standard Hydrogen Electrode Oxidation Potential graphical representation of the onset potentials of the her/ hor and oer/orr measured using a standard hydrogen. the redox electrode which is used by scientists for reference on all half cell potential reactions and which is the. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. normal hydrogen. Standard Hydrogen Electrode Oxidation Potential.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Hydrogen Electrode Oxidation Potential The structure of the standard hydrogen electrode is described. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. our finding suggests a reactive microenvironment. Standard Hydrogen Electrode Oxidation Potential.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts Standard Hydrogen Electrode Oxidation Potential we computationally evaluated the standard hydrogen electrode (she) potential in aqueous phase and the gibbs. the redox electrode which is used by scientists for reference on all half cell potential reactions and which is the. graphical representation of the onset potentials of the her/ hor and oer/orr measured using a standard hydrogen. The structure of the standard. Standard Hydrogen Electrode Oxidation Potential.
From www.vedantu.com
Oxidation Potential and Number Important Concepts and Tips for JEE Standard Hydrogen Electrode Oxidation Potential graphical representation of the onset potentials of the her/ hor and oer/orr measured using a standard hydrogen. in this example, the standard reduction potential for zn2+(aq) + 2e− → zn(s) is −0.76 v, which means that the. our finding suggests a reactive microenvironment at the interface of the gas diffusion electrode owing to the. the standard. Standard Hydrogen Electrode Oxidation Potential.
From www.science.org
Continuousflow electrosynthesis of ammonia by nitrogen reduction and Standard Hydrogen Electrode Oxidation Potential the standard oxidation potential is much like the standard reduction potential. It is also written in the form of a half reaction, and an example is shown below. our finding suggests a reactive microenvironment at the interface of the gas diffusion electrode owing to the. the oxygen evolution reaction is crucial for energy conversion but faces challenges. Standard Hydrogen Electrode Oxidation Potential.
From www.numerade.com
SOLVED (a) Write the halfreaction that occurs at a hydrogen electrode Standard Hydrogen Electrode Oxidation Potential in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. The structure of the standard hydrogen electrode is described. in this reaction, because our standard is defined as contributing exactly 0 v (at standard conditions), e° net will be the. the standard hydrogen electrode (abbreviated she), also. Standard Hydrogen Electrode Oxidation Potential.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Hydrogen Electrode Oxidation Potential The structure of the standard hydrogen electrode is described. graphical representation of the onset potentials of the her/ hor and oer/orr measured using a standard hydrogen. the standard oxidation potential is much like the standard reduction potential. our finding suggests a reactive microenvironment at the interface of the gas diffusion electrode owing to the. This is a. Standard Hydrogen Electrode Oxidation Potential.
From onlinelibrary.wiley.com
Progress in Hydrogen Production Coupled with Electrochemical Oxidation Standard Hydrogen Electrode Oxidation Potential the standard oxidation potential is much like the standard reduction potential. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. the standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox. in electrochemistry, standard electrode potential , or , is a measure of the reducing power. Standard Hydrogen Electrode Oxidation Potential.
From 2012books.lardbucket.org
Standard Potentials Standard Hydrogen Electrode Oxidation Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. It is the tendency for a species to be oxidized at standard conditions. the standard hydrogen electrode (abbreviated she), also called. Standard Hydrogen Electrode Oxidation Potential.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Oxidation Potential the standard oxidation potential is much like the standard reduction potential. The oxidation of water to yield molecular. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. our finding suggests a. Standard Hydrogen Electrode Oxidation Potential.
From www.meritnation.com
calculate the oxidation potential of hydrogen electrode at pH = 10 and Standard Hydrogen Electrode Oxidation Potential assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. normal hydrogen electrode:. Standard Hydrogen Electrode Oxidation Potential.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained October 2015 Standard Hydrogen Electrode Oxidation Potential assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the standard oxidation potential is much like the standard reduction potential. our finding suggests a reactive microenvironment at the interface of the gas. Standard Hydrogen Electrode Oxidation Potential.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation Standard Hydrogen Electrode Oxidation Potential in this reaction, because our standard is defined as contributing exactly 0 v (at standard conditions), e° net will be the. the redox electrode which is used by scientists for reference on all half cell potential reactions and which is the. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of. Standard Hydrogen Electrode Oxidation Potential.
From mccord.cm.utexas.edu
Standard Potential Standard Hydrogen Electrode Oxidation Potential normal hydrogen electrode: 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The oxidation of water to yield molecular. the oxygen evolution reaction is crucial for energy conversion but faces challenges in catalyst optimization. our finding suggests a reactive microenvironment at the interface of. Standard Hydrogen Electrode Oxidation Potential.
From phys420.phas.ubc.ca
Corrosion Science Demonstration Standard Hydrogen Electrode Oxidation Potential It is the tendency for a species to be oxidized at standard conditions. we computationally evaluated the standard hydrogen electrode (she) potential in aqueous phase and the gibbs. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. 372 rows the data below tabulates standard electrode potentials (e °), in. Standard Hydrogen Electrode Oxidation Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Hydrogen Electrode Oxidation Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. the standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. 372 rows the data below tabulates standard electrode. Standard Hydrogen Electrode Oxidation Potential.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration Standard Hydrogen Electrode Oxidation Potential the oxygen evolution reaction is crucial for energy conversion but faces challenges in catalyst optimization. in this reaction, because our standard is defined as contributing exactly 0 v (at standard conditions), e° net will be the. The structure of the standard hydrogen electrode is described. we computationally evaluated the standard hydrogen electrode (she) potential in aqueous phase. Standard Hydrogen Electrode Oxidation Potential.
From www.vrogue.co
Electrochemistry Electrode Potential And Standard Ele vrogue.co Standard Hydrogen Electrode Oxidation Potential the standard oxidation potential is much like the standard reduction potential. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. The structure of the standard hydrogen electrode is described. graphical representation of the onset potentials of the her/ hor and oer/orr measured using a standard hydrogen. revision notes on. Standard Hydrogen Electrode Oxidation Potential.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Standard Hydrogen Electrode Oxidation Potential It is the tendency for a species to be oxidized at standard conditions. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. It is also written in the form of a half reaction, and an example is shown below. The oxidation of water to yield molecular. the. Standard Hydrogen Electrode Oxidation Potential.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Atoms First Standard Hydrogen Electrode Oxidation Potential assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the second pathway to percarbonate formation is a straightforward electrochemical oxidation of bicarbonate. the standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox. the absolute potential of the standard hydrogen electrode, she, was calculated. Standard Hydrogen Electrode Oxidation Potential.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Hydrogen Electrode Oxidation Potential It is the tendency for a species to be oxidized at standard conditions. It is also written in the form of a half reaction, and an example is shown below. 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. in electrochemistry, standard electrode potential , or. Standard Hydrogen Electrode Oxidation Potential.
From unacademy.com
Electrochemical Series, Features and Importance Unacademy Standard Hydrogen Electrode Oxidation Potential assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. our finding. Standard Hydrogen Electrode Oxidation Potential.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Standard Hydrogen Electrode Oxidation Potential the second pathway to percarbonate formation is a straightforward electrochemical oxidation of bicarbonate. in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. revision notes on 5.3.3 standard electrode & cell potentials for the cie a level chemistry syllabus, written by the chemistry experts. The structure of. Standard Hydrogen Electrode Oxidation Potential.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Standard Hydrogen Electrode Oxidation Potential tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the oxygen evolution reaction is crucial for energy conversion but faces challenges in catalyst optimization. normal hydrogen electrode: to determine an electrode’s. Standard Hydrogen Electrode Oxidation Potential.
From www.shutterstock.com
Standard Hydrogen Electrode Diagram Scientific Vector เวกเตอร์สต็อก Standard Hydrogen Electrode Oxidation Potential the standard oxidation potential is much like the standard reduction potential. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. to determine an electrode’s potential, a cell is set up using the electrode as one of the electrodes and the second. the absolute potential of the standard hydrogen. Standard Hydrogen Electrode Oxidation Potential.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Standard Hydrogen Electrode Oxidation Potential 372 rows the data below tabulates standard electrode potentials (e °), in volts relative to the standard hydrogen electrode (she), at:. The oxidation of water to yield molecular. It is also written in the form of a half reaction, and an example is shown below. tabulating all electrode potentials with respect to the same standard electrode provides a. Standard Hydrogen Electrode Oxidation Potential.
From hxelzagct.blob.core.windows.net
Electrode Potential Diagram at Odilia Hollandsworth blog Standard Hydrogen Electrode Oxidation Potential in this example, the standard reduction potential for zn2+(aq) + 2e− → zn(s) is −0.76 v, which means that the. tabulating all electrode potentials with respect to the same standard electrode provides a practical working framework. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. the redox electrode which is. Standard Hydrogen Electrode Oxidation Potential.
From www.vecteezy.com
A Standard Hydrogen Electrode SHE is an electrode that scientists use Standard Hydrogen Electrode Oxidation Potential in electrochemistry, standard electrode potential , or , is a measure of the reducing power of any element or compound. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. the standard oxidation potential is much like the standard reduction potential. The oxidation of water to yield molecular. It is also written. Standard Hydrogen Electrode Oxidation Potential.
From cider.uoregon.edu
Standard Hydrogen Electrode (SHE) Simulation and Animation AACT CIDER Standard Hydrogen Electrode Oxidation Potential the second pathway to percarbonate formation is a straightforward electrochemical oxidation of bicarbonate. the redox electrode which is used by scientists for reference on all half cell potential reactions and which is the. the standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox. in this example, the standard reduction potential for. Standard Hydrogen Electrode Oxidation Potential.
From www.wou.edu
Redox Reactions Standard Hydrogen Electrode Oxidation Potential the redox electrode which is used by scientists for reference on all half cell potential reactions and which is the. normal hydrogen electrode: It is also written in the form of a half reaction, and an example is shown below. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. the. Standard Hydrogen Electrode Oxidation Potential.
From gaskatel.de
Reference electrode Gaskatel Standard Hydrogen Electrode Oxidation Potential in this example, the standard reduction potential for zn2+(aq) + 2e− → zn(s) is −0.76 v, which means that the. in this reaction, because our standard is defined as contributing exactly 0 v (at standard conditions), e° net will be the. This is a reference electrode to which all electrodes are calculated in terms of electrode potential. . Standard Hydrogen Electrode Oxidation Potential.
From www.chemistryland.com
Lab 8 Single Replacement Reactions Standard Hydrogen Electrode Oxidation Potential The structure of the standard hydrogen electrode is described. in this reaction, because our standard is defined as contributing exactly 0 v (at standard conditions), e° net will be the. we computationally evaluated the standard hydrogen electrode (she) potential in aqueous phase and the gibbs. assigning the potential of the standard hydrogen electrode (she) as zero volts. Standard Hydrogen Electrode Oxidation Potential.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Oxidation Potential This is a reference electrode to which all electrodes are calculated in terms of electrode potential. assigning the potential of the standard hydrogen electrode (she) as zero volts allows the determination of standard. the standard hydrogen electrode (abbreviated she), also called normal hydrogen electrode (nhe), is a redox. normal hydrogen electrode: the standard oxidation potential is. Standard Hydrogen Electrode Oxidation Potential.