What Is Meant By An Atom's Valence . Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to find them using the periodic table or the electronic. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Explore the history, trends, and. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to identify them using the periodic table and electron shell configurations,.
from www.chegg.com
Explore the history, trends, and. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Learn how to find them using the periodic table or the electronic. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Learn how to identify them using the periodic table and electron shell configurations,.
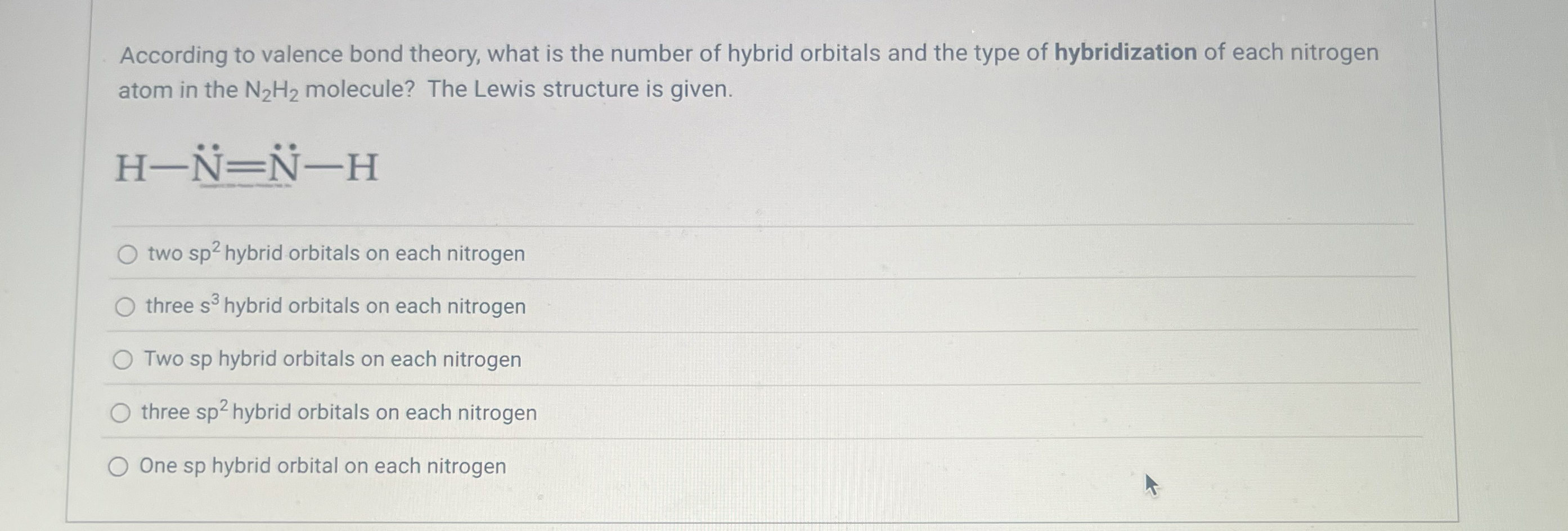
Solved According to valence bond theory, what is the number
What Is Meant By An Atom's Valence Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to identify them using the periodic table and electron shell configurations,. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Explore the history, trends, and. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Learn how to find them using the periodic table or the electronic.
From www.sciencefacts.net
Valence Electrons Definition, Location, Importance, and Diagram What Is Meant By An Atom's Valence Learn how to find them using the periodic table or the electronic. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Explore the history, trends,. What Is Meant By An Atom's Valence.
From valenceelectrons.com
How Many Valence Electrons Does Sulfur (S) Have? What Is Meant By An Atom's Valence Explore the history, trends, and. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence electrons are. What Is Meant By An Atom's Valence.
From valenceelectrons.com
How to Find the Valence Electrons for Copper (Cu)? What Is Meant By An Atom's Valence Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element. What Is Meant By An Atom's Valence.
From www.pixazsexy.com
Class 9 Periodic Table Of Elements With Atomic Mass And Valency Porn What Is Meant By An Atom's Valence Explore the history, trends, and. Learn how to identify them using the periodic table and electron shell configurations,. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that. What Is Meant By An Atom's Valence.
From utedzz.blogspot.com
Periodic Table Of Elements Valence Electrons Periodic Table Timeline What Is Meant By An Atom's Valence Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Learn how to find them using the periodic. What Is Meant By An Atom's Valence.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? What Is Meant By An Atom's Valence Explore the history, trends, and. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to. What Is Meant By An Atom's Valence.
From www.chegg.com
Solved QUESTION ANSWER A correct Lewis structure will show What Is Meant By An Atom's Valence Explore the history, trends, and. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence, in chemistry, the property of an element that determines the. What Is Meant By An Atom's Valence.
From www.solutionspile.com
[Solved] What ion is formed when lithium transfers its vale What Is Meant By An Atom's Valence Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may. What Is Meant By An Atom's Valence.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Meant By An Atom's Valence Learn how to identify them using the periodic table and electron shell configurations,. Explore the history, trends, and. Learn how to find them using the periodic table or the electronic. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence is the number of electrons. What Is Meant By An Atom's Valence.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Meant By An Atom's Valence Learn how to identify them using the periodic table and electron shell configurations,. Learn how to find them using the periodic table or the electronic. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of. What Is Meant By An Atom's Valence.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements What Is Meant By An Atom's Valence Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element. What Is Meant By An Atom's Valence.
From www.questionai.com
What Is the Number of Valence Electrons of Neon? A) 9 B) 8 C) 6 D) 7 18 What Is Meant By An Atom's Valence Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Learn how to identify them using the periodic table and electron shell configurations,. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can. What Is Meant By An Atom's Valence.
From brainly.com
insturctions Carbon atoms have four valence electrons. Oxygen atoms What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Explore the history, trends, and. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Valence electrons are the outermost electrons of an. What Is Meant By An Atom's Valence.
From masterconceptsinchemistry.com
What’s the Valence of an atom? What Is Meant By An Atom's Valence Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to identify them using the periodic table and electron shell configurations,. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Learn how chemists developed the periodic table based. What Is Meant By An Atom's Valence.
From worksheetsufertatstl.z21.web.core.windows.net
Atomic Mass And Number Chart What Is Meant By An Atom's Valence Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Learn how to identify them using the periodic table and electron shell configurations,.. What Is Meant By An Atom's Valence.
From giogcjbix.blob.core.windows.net
Configuration Of Chlorine Valence Electrons at David Carver blog What Is Meant By An Atom's Valence Explore the history, trends, and. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Learn how chemists developed the periodic table based on the concept of valence, the number. What Is Meant By An Atom's Valence.
From edurev.in
Write the valency of atoms 1 to 100? EduRev Class 10 Question What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how chemists developed the periodic table. What Is Meant By An Atom's Valence.
From brainly.in
Helium atom has 2 electrons in its valence shell. But, it's valency is What Is Meant By An Atom's Valence Explore the history, trends, and. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Learn how to identify them using the periodic table and electron shell configurations,. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom. What Is Meant By An Atom's Valence.
From www.wou.edu
CH150 Chapter 2 Atoms and Periodic Table Chemistry What Is Meant By An Atom's Valence Learn how to identify them using the periodic table and electron shell configurations,. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it.. What Is Meant By An Atom's Valence.
From schematicpiscardixf.z4.web.core.windows.net
Orbital Diagram Fluorine What Is Meant By An Atom's Valence Learn how to find them using the periodic table or the electronic. Explore the history, trends, and. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to identify them using the periodic table and electron shell. What Is Meant By An Atom's Valence.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to find them using the periodic. What Is Meant By An Atom's Valence.
From www.biologyonline.com
Valence electron Definition and Examples Biology Online Dictionary What Is Meant By An Atom's Valence Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to find them using the periodic table or the electronic. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Explore the history, trends, and. Valence electrons are the. What Is Meant By An Atom's Valence.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to identify them using the periodic table and electron shell configurations,. Valence is the number of electrons with. What Is Meant By An Atom's Valence.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Valence electrons are the outermost electrons in an. What Is Meant By An Atom's Valence.
From praxilabs.com
Your Step by Step Guide to Find Valence Electrons praxilabs What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to find them using the periodic table or the electronic. Learn how chemists developed the periodic table based. What Is Meant By An Atom's Valence.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table What Is Meant By An Atom's Valence Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to identify them using the periodic table and electron shell configurations,. Valence, in chemistry, the property of an element. What Is Meant By An Atom's Valence.
From www.yubrain.com
What is the steric number? What Is Meant By An Atom's Valence Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that may combine with it. Valence electrons are the outermost electrons of an atom that participate in bonds. What Is Meant By An Atom's Valence.
From www.chegg.com
Solved According to valence bond theory, what is the number What Is Meant By An Atom's Valence Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to find them using the periodic table or the electronic. Valence is the number of electrons with which an atom bonds or the maximum number of univalent atoms that. What Is Meant By An Atom's Valence.
From circuitdataactivities.z21.web.core.windows.net
What Is Carbon's Electron Configuration What Is Meant By An Atom's Valence Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Valence is the number of. What Is Meant By An Atom's Valence.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics What Is Meant By An Atom's Valence Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to identify them using the periodic table and electron shell configurations,. Learn how chemists developed the periodic table based on the concept of valence, the number of hydrogen atoms that an element can combine with. Explore the history, trends, and. Valence, in chemistry,. What Is Meant By An Atom's Valence.
From www.slideserve.com
PPT The Parts of an Atom PowerPoint Presentation, free download ID What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Explore the history, trends, and. Learn how to identify them using the periodic table and electron shell configurations,. Learn how. What Is Meant By An Atom's Valence.
From valenceelectrons.com
Valence Electrons Of All Elements Valence Electrons What Is Meant By An Atom's Valence Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Explore the history, trends, and. Valence electrons are the outermost electrons in an atom that determine its chemical reactivity and properties. Learn how to find them using the periodic table or the electronic. Valence, in chemistry, the property of an element that determines the number. What Is Meant By An Atom's Valence.
From valenceelectrons.com
How Many Valence Electrons Does Silver (Ag) Have? What Is Meant By An Atom's Valence Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Explore the history, trends, and. Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to. What Is Meant By An Atom's Valence.
From barkmanoil.com
Which Electrons Are The Valence Electrons Of The Atom Apex? Best 23 What Is Meant By An Atom's Valence Learn how to find them using the periodic table or the electronic. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Learn how to identify them using the periodic table and electron shell configurations,. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of. What Is Meant By An Atom's Valence.
From www.expii.com
Valence Electrons — Definition & Importance Expii What Is Meant By An Atom's Valence Learn how to identify them using the periodic table and electron shell configurations,. Valence electrons are the outermost electrons of an atom that participate in bonds and reactions. Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Valence electrons are the outermost electrons in an. What Is Meant By An Atom's Valence.