Zinc Electrons Lost . The other half of the equation involves the hydrogen ions (initially bonded to. Explore the concepts of oxidation, reduction, and half reactions in. Since the zinc atom lost electrons, it is an oxidation reaction. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. Conversely, because the oxygen atoms have gained electrons,. Because the metals have lost electrons to oxygen, they have been oxidized; In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. For example cu and cuso4. The two electrodes are connected by a electrically conducting wire. Zinc electrode is anode and copper electrode is cathode. Oxidation is therefore the loss of electrons.
from valenceelectrons.com
Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Since the zinc atom lost electrons, it is an oxidation reaction. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. Conversely, because the oxygen atoms have gained electrons,. The other half of the equation involves the hydrogen ions (initially bonded to. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Explore the concepts of oxidation, reduction, and half reactions in. For example cu and cuso4. Oxidation is therefore the loss of electrons. Because the metals have lost electrons to oxygen, they have been oxidized;
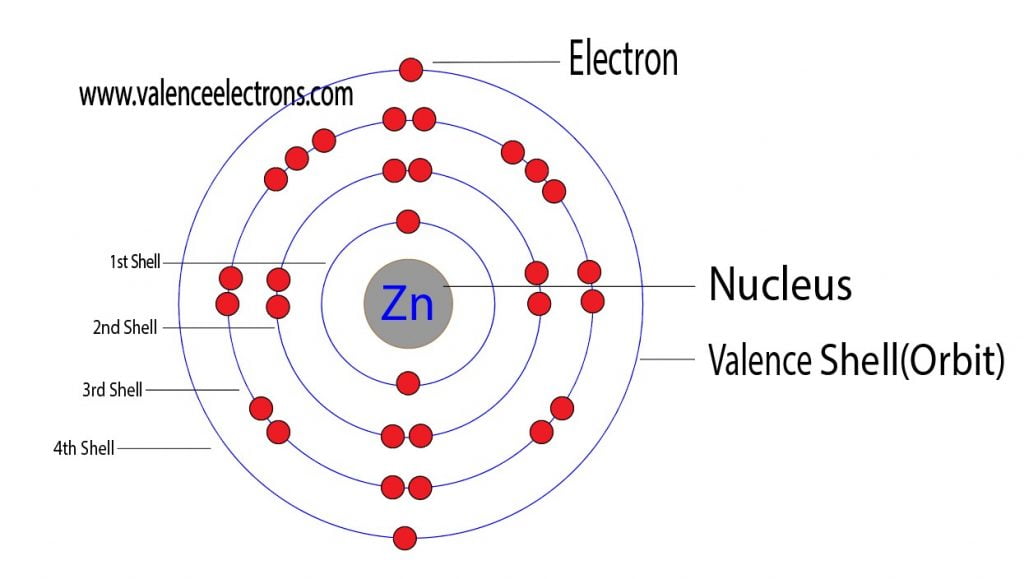
How Many Valence Electrons Does Zinc (Zn) Have?
Zinc Electrons Lost The two electrodes are connected by a electrically conducting wire. Conversely, because the oxygen atoms have gained electrons,. The other half of the equation involves the hydrogen ions (initially bonded to. Oxidation is therefore the loss of electrons. Explore the concepts of oxidation, reduction, and half reactions in. Zinc electrode is anode and copper electrode is cathode. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. For example cu and cuso4. Since the zinc atom lost electrons, it is an oxidation reaction. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. The two electrodes are connected by a electrically conducting wire. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. Because the metals have lost electrons to oxygen, they have been oxidized;
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Zinc Electrons Lost Since the zinc atom lost electrons, it is an oxidation reaction. For example cu and cuso4. Explore the concepts of oxidation, reduction, and half reactions in. Because the metals have lost electrons to oxygen, they have been oxidized; Oxidation is therefore the loss of electrons. The other half of the equation involves the hydrogen ions (initially bonded to. Learn how. Zinc Electrons Lost.
From www.pinterest.com
22 best Lessons to learn images on Pinterest School projects, Atomic Zinc Electrons Lost Conversely, because the oxygen atoms have gained electrons,. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Oxidation is therefore the loss of electrons. Because the metals have lost electrons to oxygen, they have been oxidized; Learn how zinc metal reacts with hydrochloric acid to form. Zinc Electrons Lost.
From www.nuclear-power.com
Phosphorus Electron Affinity Electronegativity Ionization Energy Zinc Electrons Lost Zinc electrode is anode and copper electrode is cathode. The other half of the equation involves the hydrogen ions (initially bonded to. For example cu and cuso4. Oxidation is therefore the loss of electrons. Because the metals have lost electrons to oxygen, they have been oxidized; In the case of a lemon battery, the ionic zinc wants to lose two. Zinc Electrons Lost.
From favpng.com
Zinc Atom Lewis Structure Bohr Model Electron Configuration, PNG Zinc Electrons Lost Conversely, because the oxygen atoms have gained electrons,. Oxidation is therefore the loss of electrons. Since the zinc atom lost electrons, it is an oxidation reaction. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Because the metals have lost electrons to oxygen, they have been. Zinc Electrons Lost.
From www.alamy.com
Symbol and electron diagram for Zinc Stock Vector Image & Art Alamy Zinc Electrons Lost Conversely, because the oxygen atoms have gained electrons,. Because the metals have lost electrons to oxygen, they have been oxidized; The two electrodes are connected by a electrically conducting wire. Zinc electrode is anode and copper electrode is cathode. Since the zinc atom lost electrons, it is an oxidation reaction. In the case of a lemon battery, the ionic zinc. Zinc Electrons Lost.
From www.numerade.com
SOLVED Write the complete electron configuration for the zinc atom Zinc Electrons Lost The other half of the equation involves the hydrogen ions (initially bonded to. Zinc electrode is anode and copper electrode is cathode. For example cu and cuso4. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Since the zinc atom lost electrons, it is an oxidation reaction. Conversely, because the oxygen. Zinc Electrons Lost.
From www.youtube.com
Electron Configuration for Zn and Zn2+ (Zinc and Zinc ion) YouTube Zinc Electrons Lost The two electrodes are connected by a electrically conducting wire. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Since the zinc atom lost electrons, it is an oxidation reaction. The other half of the equation involves the hydrogen ions (initially bonded to. For example cu. Zinc Electrons Lost.
From material-properties.org
Zinc Periodic Table and Atomic Properties Zinc Electrons Lost Zinc electrode is anode and copper electrode is cathode. Conversely, because the oxygen atoms have gained electrons,. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Oxidation is therefore the loss of electrons. Since the zinc atom lost electrons, it is an oxidation reaction. In the case of a lemon battery,. Zinc Electrons Lost.
From exofdpbhd.blob.core.windows.net
Zinc Electrons Level at Armanda Rael blog Zinc Electrons Lost Conversely, because the oxygen atoms have gained electrons,. Since the zinc atom lost electrons, it is an oxidation reaction. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them.. Zinc Electrons Lost.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Zinc Electrons Lost Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Because the metals have lost electrons to oxygen, they have been oxidized; In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. The two electrodes are connected by a. Zinc Electrons Lost.
From www.dreamstime.com
Zinc stock illustration. Illustration of render, chemistry 139650988 Zinc Electrons Lost Oxidation is therefore the loss of electrons. Since the zinc atom lost electrons, it is an oxidation reaction. The two electrodes are connected by a electrically conducting wire. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. For example cu and cuso4. Learn how zinc metal. Zinc Electrons Lost.
From www.shutterstock.com
Zinc Zn Element Sphere Electron Shell Stock Vector (Royalty Free Zinc Electrons Lost Since the zinc atom lost electrons, it is an oxidation reaction. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. The other half of the equation involves the hydrogen ions (initially bonded to. Conversely, because the oxygen atoms have gained electrons,. Explore the concepts of oxidation,. Zinc Electrons Lost.
From www.vrogue.co
Calculate The Number Of Electrons Lost Or Gained Duri vrogue.co Zinc Electrons Lost Oxidation is therefore the loss of electrons. The two electrodes are connected by a electrically conducting wire. Because the metals have lost electrons to oxygen, they have been oxidized; Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. Zinc electrode is anode and copper electrode is cathode. Since the. Zinc Electrons Lost.
From slideplayer.com
Chapter 12 Chemical Bonding by Christopher Hamaker ppt download Zinc Electrons Lost Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is therefore the loss of electrons. Conversely, because the oxygen atoms have gained electrons,. For example cu and cuso4. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Learn how zinc metal reacts with hydrochloric acid to form hydrogen. Zinc Electrons Lost.
From www.pngegg.com
Descarga gratis Configuración de electrones átomo de zinc nivel de Zinc Electrons Lost Since the zinc atom lost electrons, it is an oxidation reaction. The two electrodes are connected by a electrically conducting wire. Conversely, because the oxygen atoms have gained electrons,. Zinc electrode is anode and copper electrode is cathode. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. For example. Zinc Electrons Lost.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Zinc Electrons Lost In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Oxidation is therefore the loss of electrons. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. The other half of the equation involves the hydrogen ions. Zinc Electrons Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Lost Zinc electrode is anode and copper electrode is cathode. The two electrodes are connected by a electrically conducting wire. Conversely, because the oxygen atoms have gained electrons,. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Activity series of the metals suggest that zinc is ok. Zinc Electrons Lost.
From www.youtube.com
4.7 Ions Losing & Gaining Electrons YouTube Zinc Electrons Lost Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Oxidation is therefore the loss of electrons. Explore the concepts of oxidation, reduction, and half reactions in. Zinc electrode is anode and copper electrode is cathode. The other half of the equation involves the hydrogen ions (initially bonded to. Conversely, because the. Zinc Electrons Lost.
From socratic.org
How many unpaired electrons are in a zinc atom? Socratic Zinc Electrons Lost The other half of the equation involves the hydrogen ions (initially bonded to. Conversely, because the oxygen atoms have gained electrons,. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Since the zinc atom lost electrons, it is an oxidation reaction. Explore the concepts of oxidation,. Zinc Electrons Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Lost Explore the concepts of oxidation, reduction, and half reactions in. Zinc electrode is anode and copper electrode is cathode. Since the zinc atom lost electrons, it is an oxidation reaction. Conversely, because the oxygen atoms have gained electrons,. The two electrodes are connected by a electrically conducting wire. Oxidation is therefore the loss of electrons. Activity series of the metals. Zinc Electrons Lost.
From mungfali.com
Zinc Orbital Diagram Zinc Electrons Lost In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Zinc electrode is anode and copper electrode is cathode. The two electrodes are connected by a electrically conducting wire. Conversely, because the oxygen atoms have gained electrons,. Learn how zinc metal reacts with hydrochloric acid to form. Zinc Electrons Lost.
From www.slideserve.com
PPT Quiz Review PowerPoint Presentation, free download ID2732026 Zinc Electrons Lost Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. The two electrodes are connected by a electrically conducting wire. Since the zinc atom lost electrons, it is an oxidation reaction. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. Because. Zinc Electrons Lost.
From www.alamy.es
Configuración de electrones del zinc. Ilustración de la estructura Zinc Electrons Lost For example cu and cuso4. Zinc electrode is anode and copper electrode is cathode. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. The other half of the equation involves the hydrogen ions (initially bonded to. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride. Zinc Electrons Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Lost For example cu and cuso4. Oxidation is therefore the loss of electrons. The two electrodes are connected by a electrically conducting wire. Explore the concepts of oxidation, reduction, and half reactions in. Because the metals have lost electrons to oxygen, they have been oxidized; Zinc electrode is anode and copper electrode is cathode. In the case of a lemon battery,. Zinc Electrons Lost.
From animalia-life.club
Zinc Electron Configuration Zinc Electrons Lost Since the zinc atom lost electrons, it is an oxidation reaction. The two electrodes are connected by a electrically conducting wire. Because the metals have lost electrons to oxygen, they have been oxidized; The other half of the equation involves the hydrogen ions (initially bonded to. For example cu and cuso4. Zinc electrode is anode and copper electrode is cathode.. Zinc Electrons Lost.
From valenceelectrons.com
How to Write the Electron Configuration for Zinc (Zn)? Zinc Electrons Lost Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. The two electrodes are connected by a electrically conducting wire. The other half of the equation involves the hydrogen ions (initially bonded. Zinc Electrons Lost.
From www.youtube.com
Electrons in Atoms Lesson7 Noble Gas Configuration YouTube Zinc Electrons Lost Conversely, because the oxygen atoms have gained electrons,. Because the metals have lost electrons to oxygen, they have been oxidized; Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. The two electrodes are connected by a electrically conducting wire. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas. Zinc Electrons Lost.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Zinc Electrons Lost In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Explore the concepts of oxidation, reduction, and half reactions in. Since the zinc atom lost electrons, it is an oxidation reaction. The two electrodes are connected by a electrically conducting wire. Because the metals have lost electrons. Zinc Electrons Lost.
From valenceelectrons.com
How Many Valence Electrons Does Zinc (Zn) Have? Zinc Electrons Lost Because the metals have lost electrons to oxygen, they have been oxidized; The two electrodes are connected by a electrically conducting wire. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Explore the concepts of oxidation, reduction, and half reactions in. Learn how zinc metal reacts with hydrochloric acid to form. Zinc Electrons Lost.
From www.mooramo.com
Gaining and Losing Electrons Mooramo Zinc Electrons Lost The other half of the equation involves the hydrogen ions (initially bonded to. Since the zinc atom lost electrons, it is an oxidation reaction. Oxidation is therefore the loss of electrons. Because the metals have lost electrons to oxygen, they have been oxidized; For example cu and cuso4. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas. Zinc Electrons Lost.
From www.wou.edu
CH150 Chapter 3 Ions and Ionic Compounds Chemistry Zinc Electrons Lost Zinc electrode is anode and copper electrode is cathode. For example cu and cuso4. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. The two electrodes are connected by a electrically conducting wire. Since the zinc atom lost electrons, it is an oxidation reaction. Activity series of the metals. Zinc Electrons Lost.
From www.youtube.com
Determining the number of electrons lost or gained YouTube Zinc Electrons Lost Conversely, because the oxygen atoms have gained electrons,. Explore the concepts of oxidation, reduction, and half reactions in. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion. Oxidation is therefore the loss of electrons. Because the metals have lost electrons to oxygen, they have been oxidized; In the case of a. Zinc Electrons Lost.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Zinc Electrons Lost For example cu and cuso4. Explore the concepts of oxidation, reduction, and half reactions in. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. Because the metals have lost electrons to oxygen, they have been oxidized; Activity series of the metals suggest that zinc is ok to lose electrons. Zinc Electrons Lost.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Zinc Electrons Lost For example cu and cuso4. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Learn how zinc metal reacts with hydrochloric acid to form hydrogen gas and zinc chloride in a single replacement reaction. Conversely, because the oxygen atoms have gained electrons,. Activity series of the. Zinc Electrons Lost.
From www.mooramo.com
Ions of Transition Elements Mooramo Zinc Electrons Lost Since the zinc atom lost electrons, it is an oxidation reaction. Conversely, because the oxygen atoms have gained electrons,. In the case of a lemon battery, the ionic zinc wants to lose two electrons more than the copper wants to gain them. Activity series of the metals suggest that zinc is ok to lose electrons and turn into zinc ion.. Zinc Electrons Lost.