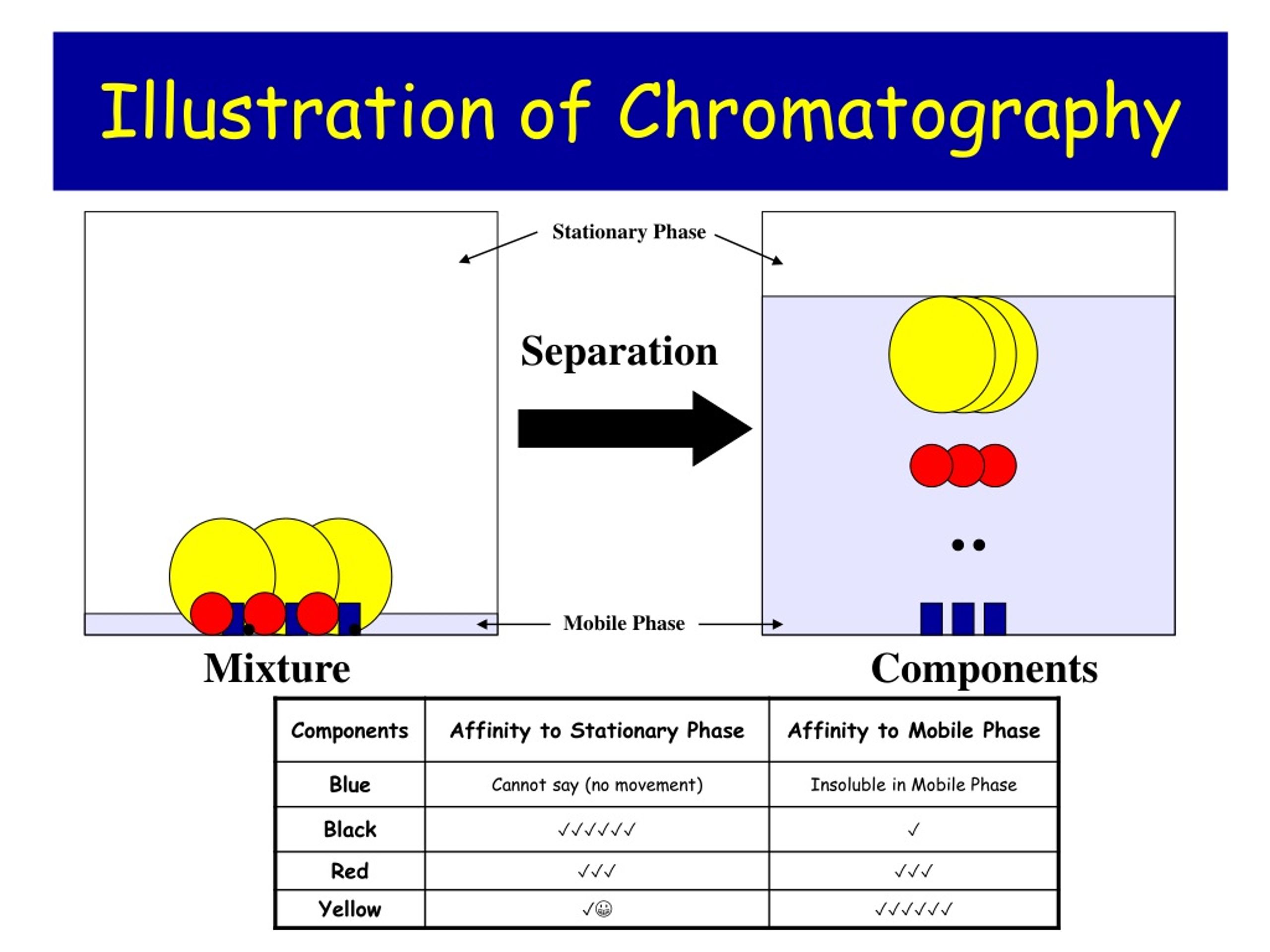
Chromatography Polarity . Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Solid, liquid, or gas can be held. The stationary phase remains fixed in place while the mobile. Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by changing the polarity of the mobile phase. These are often coloured substances such as food. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases.
from www.slideserve.com
Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by changing the polarity of the mobile phase. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. These are often coloured substances such as food. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Solid, liquid, or gas can be held. Chromatography is a separation technique used to separate mixtures of soluble substances. The stationary phase remains fixed in place while the mobile. Chromatography is regarded as one of the most significant bioanalytical techniques used in different.
PPT BASED ON POLARITY PowerPoint Presentation, free download ID1430840
Chromatography Polarity The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Solid, liquid, or gas can be held. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. These are often coloured substances such as food. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. The stationary phase remains fixed in place while the mobile. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is a separation technique used to separate mixtures of soluble substances. Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by changing the polarity of the mobile phase.
From mungfali.com
Thin Layer Chromatography Polarity Chromatography Polarity The stationary phase remains fixed in place while the mobile. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. These are often coloured substances such as food. Chromatography is a separation technique used to separate mixtures of soluble substances. Solid, liquid, or gas can be. Chromatography Polarity.
From www.youtube.com
Column Chromatography (Polarity Based Chromatography) Methods in Chromatography Polarity The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. These are often coloured substances such as food. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary. Chromatography Polarity.
From www.numerade.com
SOLVED Thin layer chromatography works on the same polarity principles Chromatography Polarity Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Solid, liquid, or gas can be held. Chromatography is. Chromatography Polarity.
From www.youtube.com
Molecular Polarity used in Chromatography YouTube Chromatography Polarity Solid, liquid, or gas can be held. The stationary phase remains fixed in place while the mobile. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is a method by which a mixture is separated by distributing its components between two phases. These are often coloured substances such as food. Chromatography is a separation. Chromatography Polarity.
From travelintheworld.ga
Paper chromatography polarity Chromatography Polarity The stationary phase remains fixed in place while the mobile. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. The stationary phase is a crucial component of chromatography that interacts with. Chromatography Polarity.
From www.slideshare.net
Chromatography Chromatography Polarity Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by changing the polarity of the mobile phase. Solid, liquid, or gas can be held. Chromatography is a separation technique used to separate mixtures of soluble substances. The ability of chromatography to separate components in a mixture depends on equilibration. Chromatography Polarity.
From www.slideserve.com
PPT Column Chromatography PowerPoint Presentation, free download ID Chromatography Polarity The stationary phase remains fixed in place while the mobile. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Solid, liquid, or gas can be held. Chromatography is a method by. Chromatography Polarity.
From www.learnatnoon.com
What is Chromatography? Noon Academy Chromatography Polarity Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile. Chromatography is a separation technique used to separate mixtures of soluble substances. Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by. Chromatography Polarity.
From travistra.blogspot.com
COLUMN Chromatography Polarity Solid, liquid, or gas can be held. These are often coloured substances such as food. The stationary phase remains fixed in place while the mobile. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface. Chromatography Polarity.
From www.slideserve.com
PPT Chemistry 4631 PowerPoint Presentation, free download ID5429136 Chromatography Polarity Chromatography is a method by which a mixture is separated by distributing its components between two phases. These are often coloured substances such as food. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is effective because different components within a mixture. Chromatography Polarity.
From www.youtube.com
HPLC Reversedphase liquid chromatography animation RPLC YouTube Chromatography Polarity Solid, liquid, or gas can be held. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. These are often coloured substances such as food. The stationary phase remains fixed in place while the mobile. The stationary phase is a crucial component of chromatography that interacts. Chromatography Polarity.
From www.slideserve.com
PPT classification of chromatography PowerPoint Presentation, free Chromatography Polarity Solid, liquid, or gas can be held. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Since the mobile phase is an important factor, it is possible to change the rf. Chromatography Polarity.
From www.slideserve.com
PPT BASED ON POLARITY PowerPoint Presentation, free download ID1430840 Chromatography Polarity The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. These are often coloured substances such as food. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the. Chromatography Polarity.
From lab-training.com
Ion pair chromatography in reverse phase HPLC Chromatography Polarity Solid, liquid, or gas can be held. These are often coloured substances such as food. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. The ability of chromatography to separate components in a mixture depends. Chromatography Polarity.
From www.vrogue.co
Types Of Chromatography Used In Bioprocessing Design vrogue.co Chromatography Polarity Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by changing the polarity of the mobile phase. Chromatography is a separation technique used to separate mixtures of soluble substances. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation.. Chromatography Polarity.
From www.youtube.com
Polarity Demonstration Paper Chromatography Video 2 YouTube Chromatography Polarity The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. The ability of chromatography to separate components in a mixture depends on equilibration of a compound. Chromatography Polarity.
From www.slideserve.com
PPT BASED ON POLARITY PowerPoint Presentation, free download ID1430840 Chromatography Polarity Solid, liquid, or gas can be held. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. These are often coloured substances such as food. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending. Chromatography Polarity.
From mavink.com
Thin Layer Chromatography Polarity Chromatography Polarity Solid, liquid, or gas can be held. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. These are often coloured substances such as food. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile. Chromatography Polarity.
From www.slideshare.net
Chromatography Chromatography Polarity Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Chromatography is a separation technique used to separate mixtures of soluble. Chromatography Polarity.
From slidetodoc.com
Colors in Chemistry Learning Polarity with Paper Chromatography Chromatography Polarity These are often coloured substances such as food. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Solid, liquid, or gas can be held. Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by. Chromatography Polarity.
From www.researchgate.net
Thin layer chromatography plate showing the polar lipid profile of (a Chromatography Polarity These are often coloured substances such as food. Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by changing the polarity of the mobile phase. The stationary phase remains fixed in place while the mobile. Solid, liquid, or gas can be held. Chromatography is a method by which a. Chromatography Polarity.
From slideplayer.com
Chemistry Work on “Chem Phys Properties” Today ppt download Chromatography Polarity The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. The stationary phase remains fixed in place while the mobile. Solid, liquid, or gas can be. Chromatography Polarity.
From www.slideserve.com
PPT classification of chromatography PowerPoint Presentation ID7395851 Chromatography Polarity Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. The stationary phase remains fixed in place while the. Chromatography Polarity.
From chemistryhall.com
Thin Layer Chromatography A Complete Guide to TLC Chromatography Polarity Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Solid, liquid, or gas can be held. The stationary phase remains fixed in place while the mobile. These are often coloured substances such as food. The ability of chromatography. Chromatography Polarity.
From www.slideserve.com
PPT Experiment 3.7 Solvent and Polarity Effects in Thinlayer Chromatography Polarity The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. These are often coloured substances such as food. Chromatography is a separation technique used to separate mixtures of soluble substances. Solid, liquid, or gas. Chromatography Polarity.
From www.slideserve.com
PPT Chromatography PowerPoint Presentation, free download ID3406205 Chromatography Polarity Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is a method by which a mixture is separated by distributing its components. Chromatography Polarity.
From www.slideserve.com
PPT Ch 28 Liquid Chromatography PowerPoint Presentation, free Chromatography Polarity The stationary phase remains fixed in place while the mobile. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Solid, liquid, or gas can be held. Chromatography is effective because different components within. Chromatography Polarity.
From studyingchemistry.tumblr.com
Life Of A Chemist Column chromatography Chromatography Polarity Solid, liquid, or gas can be held. These are often coloured substances such as food. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is effective because different components within a mixture are attracted to the adsorbent. Chromatography Polarity.
From slidetodoc.com
Colors in Chemistry Learning Polarity with Paper Chromatography Chromatography Polarity Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. The stationary phase remains fixed in place while the mobile. These are often coloured substances such as food. The stationary phase is a crucial. Chromatography Polarity.
From www.researchgate.net
Twodimensional thinlayer chromatography of polar lipids of strains Chromatography Polarity These are often coloured substances such as food. The stationary phase is a crucial component of chromatography that interacts with analytes as they pass through, leading to separation. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Solid,. Chromatography Polarity.
From blog.sepscience.com
Basis of Interactions in Gas Chromatography Part 2 Polar Interactions Chromatography Polarity Chromatography is a method by which a mixture is separated by distributing its components between two phases. The stationary phase remains fixed in place while the mobile. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. These are often coloured substances such as food. Since the mobile. Chromatography Polarity.
From www.youtube.com
Paper Chromatography Intro & Theory YouTube Chromatography Polarity Solid, liquid, or gas can be held. The stationary phase remains fixed in place while the mobile. These are often coloured substances such as food. Chromatography is a separation technique used to separate mixtures of soluble substances. Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is effective because different components. Chromatography Polarity.
From www.slideserve.com
PPT Chapter 28 Liquid Chromatography PowerPoint Presentation, free Chromatography Polarity The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Chromatography is a method by which a mixture is separated by distributing its components. Chromatography Polarity.
From mavink.com
Gc Column Polarity Chart Chromatography Polarity Chromatography is a method by which a mixture is separated by distributing its components between two phases. Chromatography is a separation technique used to separate mixtures of soluble substances. Since the mobile phase is an important factor, it is possible to change the rf r f of a compound by changing the polarity of the mobile phase. These are often. Chromatography Polarity.
From microbiologynotes.org
Different Types of chromatography Microbiology Notes Chromatography Polarity The stationary phase remains fixed in place while the mobile. Chromatography is regarded as one of the most significant bioanalytical techniques used in different. Chromatography is effective because different components within a mixture are attracted to the adsorbent surface of the stationary phase with varying degrees depending on. Chromatography is a method by which a mixture is separated by distributing. Chromatography Polarity.