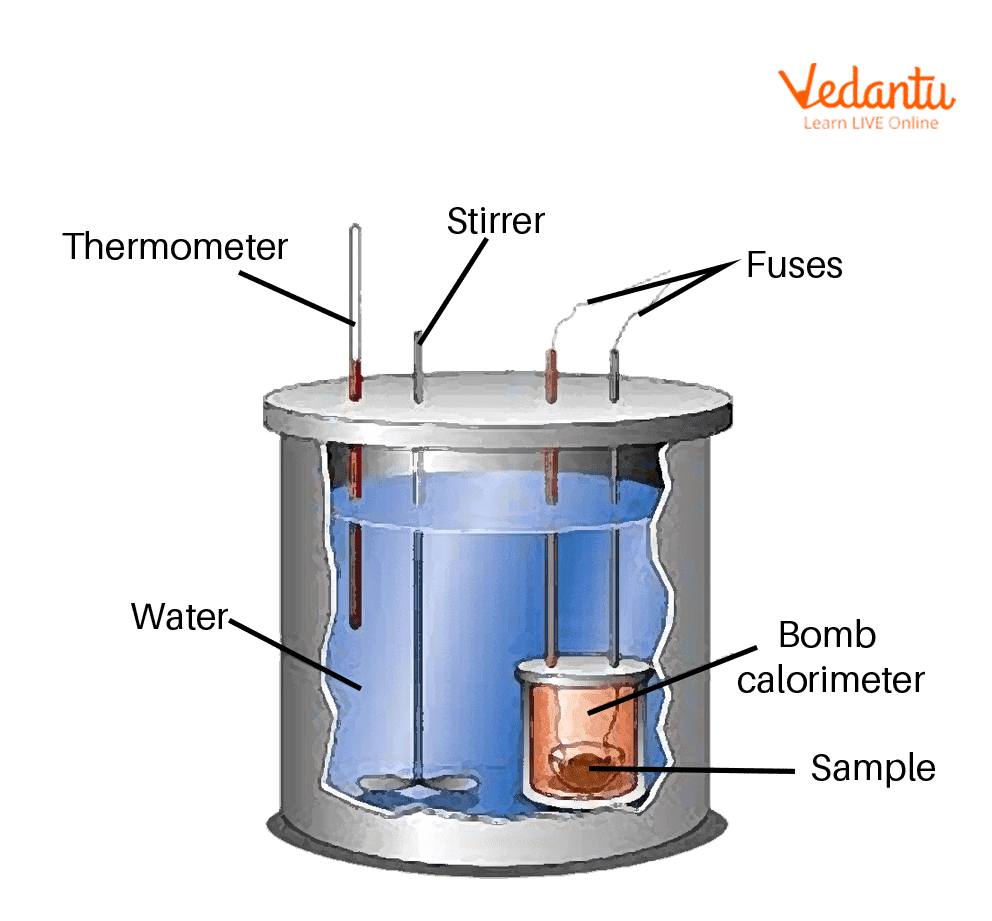
Bomb Calorimeter Is Used To Determine Enthalpy . Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The principle of operation is to saturate the material. The emitted heat increases the temperature of the water. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are.
from www.vedantu.com
Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. The emitted heat increases the temperature of the water. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. The principle of operation is to saturate the material. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and.
Bomb Calorimeter Learn Important Terms and Concepts
Bomb Calorimeter Is Used To Determine Enthalpy A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. The emitted heat increases the temperature of the water. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The principle of operation is to saturate the material. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Bomb Calorimeter Is Used To Determine Enthalpy A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 Bomb Calorimeter Is Used To Determine Enthalpy The principle of operation is to saturate the material. Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The emitted heat increases the temperature of the water. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. The. Bomb Calorimeter Is Used To Determine Enthalpy.
From shaunmwilliams.com
Chapter 6 Presentation Bomb Calorimeter Is Used To Determine Enthalpy A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.labster.com
5 Ways to Make Calorimetry and the Bomb Calorimeter More Approachable Bomb Calorimeter Is Used To Determine Enthalpy Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The emitted heat increases the temperature of the water. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. A bomb calorimeter is an instrument used to determine the. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.youtube.com
Bomb Calorimeter_Methodology and Solved Numerical _Fuel and its Bomb Calorimeter Is Used To Determine Enthalpy The emitted heat increases the temperature of the water. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The bomb calorimeter shown in figure 61.5 has. Bomb Calorimeter Is Used To Determine Enthalpy.
From quiztricksters.z21.web.core.windows.net
How To Calculate Calorimeter Bomb Calorimeter Is Used To Determine Enthalpy The emitted heat increases the temperature of the water. Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Is Used To Determine Enthalpy Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: The emitted heat increases the temperature of the water. Approximately one gramme of sample fuel is ground and diluted after each test to fit into. Bomb Calorimeter Is Used To Determine Enthalpy.
From classnotes.org.in
Measurement Of Change In Internal Energy and Enthalpy Chemistry Bomb Calorimeter Is Used To Determine Enthalpy The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The emitted heat increases the temperature of the water. The principle of operation is to saturate the material. A bomb calorimeter is a device used to. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Bomb Calorimeter Is Used To Determine Enthalpy Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured. Bomb Calorimeter Is Used To Determine Enthalpy.
From thermonine92.blogspot.com
Thermochemistry Calorimeter Bomb Calorimeter Is Used To Determine Enthalpy The emitted heat increases the temperature of the water. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Approximately one gramme of sample fuel is ground and. Bomb Calorimeter Is Used To Determine Enthalpy.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Bomb Calorimeter Is Used To Determine Enthalpy The emitted heat increases the temperature of the water. Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. A bomb calorimeter is an instrument used to. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Bomb Calorimeter Is Used To Determine Enthalpy The emitted heat increases the temperature of the water. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that. Bomb Calorimeter Is Used To Determine Enthalpy.
From chemlab.truman.edu
Parr 1341 Bomb Calorimeter Chem Lab Bomb Calorimeter Is Used To Determine Enthalpy Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: The principle of operation is to saturate the material. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. Constant volume calorimetry, also. Bomb Calorimeter Is Used To Determine Enthalpy.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses Bomb Calorimeter Is Used To Determine Enthalpy The emitted heat increases the temperature of the water. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. The principle of operation is. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.youtube.com
MECHANICAL ENGINEERING THE BOMB CALORIMETER IS AN APPARATUS TO MEASURE Bomb Calorimeter Is Used To Determine Enthalpy Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The principle of operation is to saturate the material. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.slideserve.com
PPT Thermodynamics PowerPoint Presentation, free download ID2691782 Bomb Calorimeter Is Used To Determine Enthalpy The principle of operation is to saturate the material. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. The bomb calorimeter shown in. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.studypool.com
SOLUTION Bomb calorimeter study material Studypool Bomb Calorimeter Is Used To Determine Enthalpy Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The emitted heat increases the temperature of the water. A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. The bomb calorimeter shown in figure. Bomb Calorimeter Is Used To Determine Enthalpy.
From ar.inspiredpencil.com
Bomb Calorimeter Styrofoam Cup Bomb Calorimeter Is Used To Determine Enthalpy The emitted heat increases the temperature of the water. The principle of operation is to saturate the material. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Bomb calorimetry is generally used to measure the heat of combustion for organic. Bomb Calorimeter Is Used To Determine Enthalpy.
From pubs.sciepub.com
Figure 1. Diagram of Bomb Calorimeter used in this Study Measuring Bomb Calorimeter Is Used To Determine Enthalpy Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. A bomb calorimeter is an instrument used to determine the heat. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.slideserve.com
PPT Chapter 6 Thermochemistry PowerPoint Presentation, free download Bomb Calorimeter Is Used To Determine Enthalpy The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. Bomb calorimetry is used. Bomb Calorimeter Is Used To Determine Enthalpy.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Bomb Calorimeter Is Used To Determine Enthalpy The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The emitted heat increases the temperature of the water. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Bomb Calorimeter Is Used To Determine Enthalpy Bomb calorimetry is generally used to measure the heat of combustion for organic materials. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. The principle of operation is to saturate the material. Approximately one gramme of sample fuel is ground and diluted after each test to fit. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.chemistryland.com
Enthalpy Bomb Calorimeter Is Used To Determine Enthalpy The principle of operation is to saturate the material. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. The emitted heat increases the temperature of the water. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and.. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.mdpi.com
Processes Free FullText Predicting Enthalpy of Combustion Using Bomb Calorimeter Is Used To Determine Enthalpy The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.alamy.com
Mahler bomb calorimeter, a device used to determine the high calorific Bomb Calorimeter Is Used To Determine Enthalpy The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. Constant volume calorimetry, also know as bomb calorimetry, is used to. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.youtube.com
How to Calculate Enthalpy Change Using a Calorimeter YouTube Bomb Calorimeter Is Used To Determine Enthalpy Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.youtube.com
Measuring Energy at Constant Volume Using a Bomb Calorimeter YouTube Bomb Calorimeter Is Used To Determine Enthalpy The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. The emitted heat increases the temperature of the water. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.thoughtco.com
Calorimeter Definition in Chemistry Bomb Calorimeter Is Used To Determine Enthalpy Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. The principle of operation is. Bomb Calorimeter Is Used To Determine Enthalpy.
From 2012books.lardbucket.org
Calorimetry Bomb Calorimeter Is Used To Determine Enthalpy Bomb calorimetry is generally used to measure the heat of combustion for organic materials. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity of biomass sample combustion and to calculate the hhv of that biomass fuel. The principle of operation is to saturate the material. The heat of formation of naphthalene and the. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Bomb Calorimeter Is Used To Determine Enthalpy The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: Bomb calorimetry is. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.youtube.com
Bomb Calorimeter Working and Principle of Bomb Calorimeter GCV of Bomb Calorimeter Is Used To Determine Enthalpy Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The emitted heat increases the temperature of the water. The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. A bomb calorimeter is an instrument used to determine. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Bomb Calorimeter Is Used To Determine Enthalpy Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: Bomb calorimetry is generally used to measure the heat of combustion for organic materials. Approximately one gramme of sample fuel is ground and diluted after each test to fit into a capsule for bomb combustion. The bomb calorimeter shown in figure 61.5 has been constructed for measuring. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Bomb Calorimeter Is Used To Determine Enthalpy The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. A bomb calorimeter is an instrument used to determine the heat emitted from a given quantity. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby Bomb Calorimeter Is Used To Determine Enthalpy The bomb calorimeter shown in figure 61.5 has been constructed for measuring the enthalpy of combustion of compounds in oxygen, which are. Constant volume calorimetry, also know as bomb calorimetry, is used to measure the heat of a reaction while holding volume constant and. The emitted heat increases the temperature of the water. A bomb calorimeter is an instrument used. Bomb Calorimeter Is Used To Determine Enthalpy.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool Bomb Calorimeter Is Used To Determine Enthalpy The heat of formation of naphthalene and the ‘unknown’ compound can be determined from its experimentally measured heat of combustion. Bomb calorimetry is used to determine the enthalpy of combustion, dcombh, for hydrocarbons: A bomb calorimeter is a device used to measure the heat of combustion of fuels, the enthalpy change of reactions, the heat capacity of. Bomb calorimetry is. Bomb Calorimeter Is Used To Determine Enthalpy.