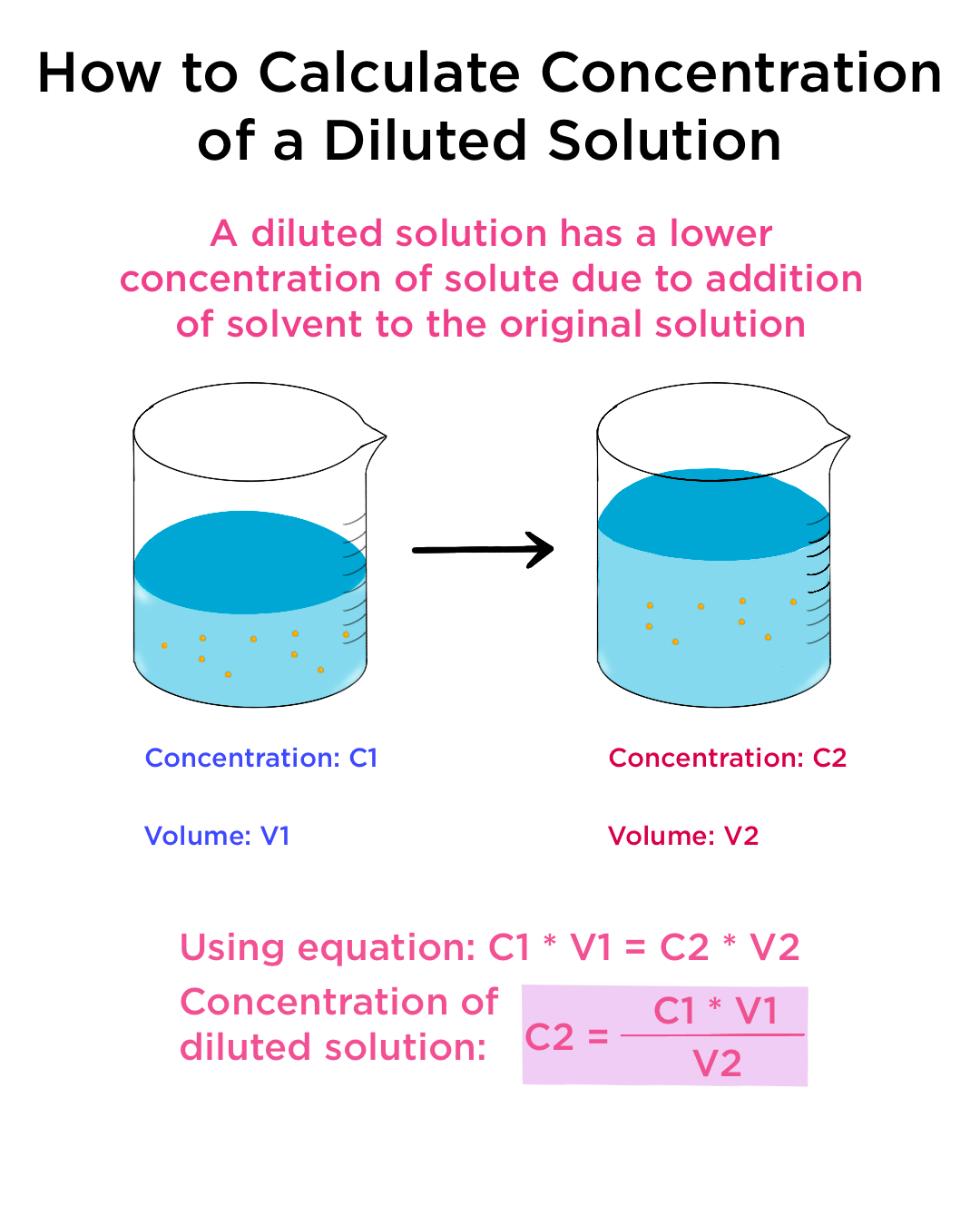
Dilution Equation Examples . C 1 v 1 = c 2 v 2 where: V1 is the volume of the starting solution. C1 is the concentration of the starting solution. you can calculate the concentration of a solution following a dilution by applying this equation: this yields the most general form of the dilution equation: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution.
from www.expii.com
\[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. C1 is the concentration of the starting solution. C 1 v 1 = c 2 v 2 where: apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. this yields the most general form of the dilution equation: V1 is the volume of the starting solution. you can calculate the concentration of a solution following a dilution by applying this equation: to make a fixed amount of a dilute solution from a stock solution, you can use the formula:
Dilution of Solutions — Overview & Examples Expii
Dilution Equation Examples V1 is the volume of the starting solution. C1 is the concentration of the starting solution. you can calculate the concentration of a solution following a dilution by applying this equation: apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. V1 is the volume of the starting solution. this yields the most general form of the dilution equation: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C 1 v 1 = c 2 v 2 where: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution Equation Examples V1 is the volume of the starting solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. M i v i = m f v f where m is molarity, v is volume, and. Dilution Equation Examples.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilution Equation Examples C1 is the concentration of the starting solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1. Dilution Equation Examples.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Dilution Equation Examples you can calculate the concentration of a solution following a dilution by applying this equation: C1 is the concentration of the starting solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C 1 v 1 = c 2 v 2 where: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v. Dilution Equation Examples.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Equation Examples \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. V1 is the volume of the starting solution. C1 is the concentration of the starting solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. you can calculate the concentration of a solution following a dilution by applying this. Dilution Equation Examples.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Equation Examples C1 is the concentration of the starting solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. you can calculate the concentration of a solution following a dilution by applying this equation: to make a fixed amount of a dilute solution from a stock solution, you can use the formula: V1 is the. Dilution Equation Examples.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Equation Examples to make a fixed amount of a dilute solution from a stock solution, you can use the formula: C 1 v 1 = c 2 v 2 where: this yields the most general form of the dilution equation: you can calculate the concentration of a solution following a dilution by applying this equation: M i v i. Dilution Equation Examples.
From www.youtube.com
Dilution in biology lab How to use the equation C1V1 = C2V2 YouTube Dilution Equation Examples V1 is the volume of the starting solution. C 1 v 1 = c 2 v 2 where: C1 is the concentration of the starting solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. \[c_1v_1=c_2v_2\] to summarize, c stands. Dilution Equation Examples.
From www.youtube.com
A Level Chemistry Dilution Calculations Worked Example YouTube Dilution Equation Examples apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. C1 is the concentration of the starting solution. this yields the most general form of the dilution equation: the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. the. Dilution Equation Examples.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Equation Examples V1 is the volume of the starting solution. you can calculate the concentration of a solution following a dilution by applying this equation: the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. C 1 v 1 = c 2 v 2 where: C1 is the concentration of. Dilution Equation Examples.
From www.slideshare.net
Molarity and dilution Dilution Equation Examples \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: you can calculate the concentration of a solution following a dilution by applying this equation: C 1 v 1 = c 2 v 2 where:. Dilution Equation Examples.
From chem2u.blogspot.com
chem2U Dilution method formula Dilution Equation Examples C1 is the concentration of the starting solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C 1 v 1 = c 2 v 2 where: to make a fixed amount of a dilute solution from a stock solution, you can use the formula: this yields the most general form of the. Dilution Equation Examples.
From www.scribd.com
Dilution (Equation) Wikipedia PDF Solution Chemistry Dilution Equation Examples to make a fixed amount of a dilute solution from a stock solution, you can use the formula: you can calculate the concentration of a solution following a dilution by applying this equation: C1 is the concentration of the starting solution. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. V1 is. Dilution Equation Examples.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Equation Examples M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: you can calculate the concentration of a solution following a dilution. Dilution Equation Examples.
From www.youtube.com
DILUTION AND MIXING OF SOLUTIONS, MOLARITY EQUATION /SOME BASIC Dilution Equation Examples C1 is the concentration of the starting solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: the dilution formula. Dilution Equation Examples.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Equation Examples the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. V1 is the volume of the starting solution. C 1 v 1 = c 2 v 2 where: apply the dilution equation to calculate the final concentration, or the final. Dilution Equation Examples.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation Examples apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. C1 is the concentration of the starting. Dilution Equation Examples.
From www.youtube.com
Chem121 Dilution Equation 8 5 YouTube Dilution Equation Examples M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. this yields the most general form of the dilution equation: you can calculate the concentration of a solution following a dilution by applying this equation: C1 is the concentration. Dilution Equation Examples.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Equation Examples \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. this yields the most general. Dilution Equation Examples.
From www.ck12.org
Dilution (M[i]V[i]=M[f]V[f]) Example 1 ( Video ) Chemistry CK12 Dilution Equation Examples \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. to. Dilution Equation Examples.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution Equation Examples you can calculate the concentration of a solution following a dilution by applying this equation: this yields the most general form of the dilution equation: the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. M i v i = m f v f where m is. Dilution Equation Examples.
From www.youtube.com
DilutionTitration Calculation ChemCram 101 Tutorial YouTube Dilution Equation Examples C 1 v 1 = c 2 v 2 where: you can calculate the concentration of a solution following a dilution by applying this equation: apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. the. Dilution Equation Examples.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision Dilution Equation Examples V1 is the volume of the starting solution. C 1 v 1 = c 2 v 2 where: this yields the most general form of the dilution equation: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. you can calculate the concentration of a solution following a dilution by applying this equation:. Dilution Equation Examples.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Equation Examples V1 is the volume of the starting solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. C 1 v 1 = c 2 v 2 where: you can. Dilution Equation Examples.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Equation Examples V1 is the volume of the starting solution. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: you can calculate the concentration of a solution following a dilution by applying this equation: the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a. Dilution Equation Examples.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Equation Examples the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C1 is the concentration of the starting solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. this yields the most general form of the dilution equation: apply the dilution equation to. Dilution Equation Examples.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Equation Examples \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. this yields the most general form of the dilution equation: to make a fixed amount of a dilute solution from a stock solution, you can use the formula: you can calculate the concentration of a solution following a dilution by applying this. Dilution Equation Examples.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Equation Examples you can calculate the concentration of a solution following a dilution by applying this equation: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. to make a fixed amount of a dilute solution from a stock solution, you. Dilution Equation Examples.
From www.youtube.com
Dilution problems Chemistry Molarity & Concentration Examples Dilution Equation Examples M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. you can calculate the concentration of a solution following a dilution by applying this equation: apply the dilution equation to calculate the final concentration, or the final volume, of. Dilution Equation Examples.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Concentration Dilution Equation Examples apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. you can calculate the concentration of a solution following a dilution by. Dilution Equation Examples.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution Equation Examples M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. you can calculate the concentration of a solution following a dilution by applying this equation:. Dilution Equation Examples.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog Dilution Equation Examples you can calculate the concentration of a solution following a dilution by applying this equation: apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. the dilution formula (c1v1 = c2v2) calculates how to mix a concentrated solution (c1v1) with a solvent to achieve a. \[c_1v_1=c_2v_2\] to summarize, c stands. Dilution Equation Examples.
From www.youtube.com
Chemistry 11 Dilution Calculations Solving for Initial Volume Example Dilution Equation Examples V1 is the volume of the starting solution. apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume. to make a fixed amount of a dilute solution from a stock solution, you can use the formula:. Dilution Equation Examples.
From www.slideshare.net
Molarity and dilution Dilution Equation Examples to make a fixed amount of a dilute solution from a stock solution, you can use the formula: the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C1 is the concentration of the starting solution. you can calculate the concentration of a solution following a dilution by applying this equation: \[c_1v_1=c_2v_2\] to summarize,. Dilution Equation Examples.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe Dilution Equation Examples C1 is the concentration of the starting solution. you can calculate the concentration of a solution following a dilution by applying this equation: the formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. to make a fixed amount of a dilute solution from a stock solution, you can use the formula: the dilution. Dilution Equation Examples.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Equation Examples you can calculate the concentration of a solution following a dilution by applying this equation: apply the dilution equation to calculate the final concentration, or the final volume, of a diluted solution. this yields the most general form of the dilution equation: V1 is the volume of the starting solution. C 1 v 1 = c 2. Dilution Equation Examples.