Pda Guidelines For Tunnel Validation . this technical report is an update of pda’s technical report no. 3, validation of dry heat processes used for sterilization and. 3, originally issued in 1981. The very first technical guidance written by pda. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. a core area of focus for pda’s membership is sterilization of pharmaceutical products. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. pda has revised technical report no.
from johnsonfrancis.org
pda has revised technical report no. 3, originally issued in 1981. 3, validation of dry heat processes used for sterilization and. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. this technical report is an update of pda’s technical report no. a core area of focus for pda’s membership is sterilization of pharmaceutical products. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. The very first technical guidance written by pda.
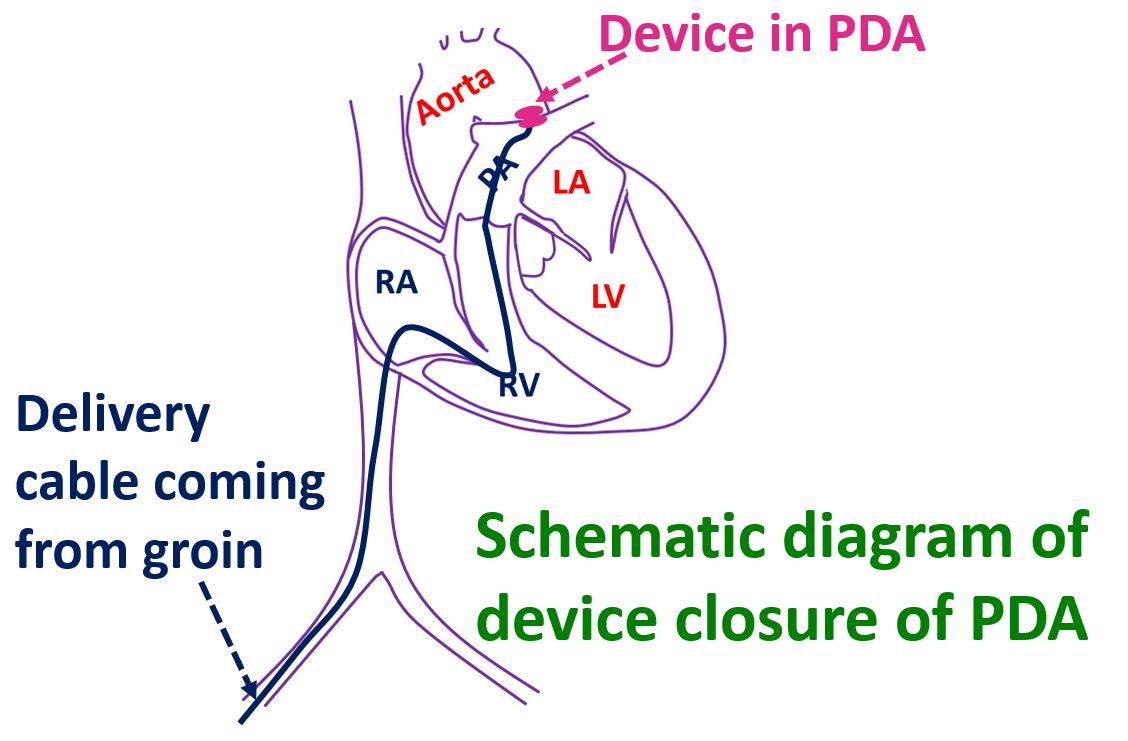
Device closure of PDA All About Heart And Blood Vessels
Pda Guidelines For Tunnel Validation this technical report is an update of pda’s technical report no. 3, originally issued in 1981. a core area of focus for pda’s membership is sterilization of pharmaceutical products. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. 3, validation of dry heat processes used for sterilization and. The very first technical guidance written by pda. this technical report is an update of pda’s technical report no. pda has revised technical report no. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing.
From www.guidelinegeo.com
Tunnel Inspection Guideline Geo GPR SEISMICS Pda Guidelines For Tunnel Validation 3, validation of dry heat processes used for sterilization and. pda has revised technical report no. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. this technical report is an update of pda’s technical report no. 3, originally issued in 1981. the sterilizing & depyrogenating. Pda Guidelines For Tunnel Validation.
From link.springer.com
Development and validation of a patient decision aid for prostate Pda Guidelines For Tunnel Validation The very first technical guidance written by pda. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. this technical report is an update of pda’s technical report no. 3, validation of dry. Pda Guidelines For Tunnel Validation.
From dokumen.tips
(PDF) Digitalized tunnel face mapping system (DiTFAMS) using PDA and Pda Guidelines For Tunnel Validation the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. pda has revised technical report no. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. this technical report is an update of pda’s technical report no. 3, originally issued in 1981.. Pda Guidelines For Tunnel Validation.
From www.scirp.org
Carpal tunnel syndrome diagnosis validation of a clinicbased nerve Pda Guidelines For Tunnel Validation pda has revised technical report no. The very first technical guidance written by pda. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. a core area of focus for pda’s membership is sterilization of pharmaceutical products. 3, validation of dry heat processes used for sterilization and. the purpose of this protocol. Pda Guidelines For Tunnel Validation.
From valimation.com
A RiskBased Approach to ERP Systems Validation Published in PDA Pda Guidelines For Tunnel Validation The very first technical guidance written by pda. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. 3, originally issued in 1981. this technical report is an update of pda’s technical report no. 3, validation of dry heat processes used for sterilization and. pda has revised. Pda Guidelines For Tunnel Validation.
From www.usvalidation.com
Depyrogenation Oven and Tunnel Sterilizer Validation Pda Guidelines For Tunnel Validation 3, validation of dry heat processes used for sterilization and. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. a core area of focus for pda’s membership is sterilization of pharmaceutical products. this technical report is an update of pda’s technical report no. pda has revised technical report no. The very. Pda Guidelines For Tunnel Validation.
From www.slideshare.net
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION Pda Guidelines For Tunnel Validation the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. 3, originally issued in 1981. this technical report is an update of pda’s technical report no. The very first technical guidance written by. Pda Guidelines For Tunnel Validation.
From www.midasbridge.com
Guidelines for Tunnel Design Pda Guidelines For Tunnel Validation the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. this technical report is an update of pda’s technical report no. 3, originally issued in 1981. 3, validation of dry heat processes used for sterilization and. pda has revised technical report no. a core area of focus for pda’s membership is sterilization. Pda Guidelines For Tunnel Validation.
From searchforhappiness.eu
DEPYROGENATION TUNNEL VALIDATION PDF Pda Guidelines For Tunnel Validation pda has revised technical report no. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. this technical report is an update of pda’s technical report no. 3, originally issued in 1981.. Pda Guidelines For Tunnel Validation.
From exoudvpek.blob.core.windows.net
Pda Guidelines For Visual Inspection at Courtney Klein blog Pda Guidelines For Tunnel Validation pda has revised technical report no. a core area of focus for pda’s membership is sterilization of pharmaceutical products. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. The very first technical guidance written by pda. 3, validation of dry heat processes used for sterilization and. this technical report is an. Pda Guidelines For Tunnel Validation.
From www.clinicalguidelines.scot.nhs.uk
Patent ductus arteriosus (PDA) surgical ligation Pda Guidelines For Tunnel Validation The very first technical guidance written by pda. 3, originally issued in 1981. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. pda has revised technical report no. 3, validation of dry heat processes used for sterilization and. the sterilizing & depyrogenating tunnel when operated with. Pda Guidelines For Tunnel Validation.
From johnsonfrancis.org
Device closure of PDA All About Heart And Blood Vessels Pda Guidelines For Tunnel Validation the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. pda has revised technical report no. a core area of focus for pda’s membership is sterilization of pharmaceutical products. 3, validation of dry heat processes used for sterilization and. the purpose of this protocol is to provide an outline for the qualification. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
Validation parameters obtained with PA and PDA fibers, respectively Pda Guidelines For Tunnel Validation this technical report is an update of pda’s technical report no. 3, originally issued in 1981. 3, validation of dry heat processes used for sterilization and. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. a core area of focus for pda’s membership is sterilization of pharmaceutical products. the purpose of. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
(PDF) RPHPLCPDA Method Development, Validation and Stability Studies Pda Guidelines For Tunnel Validation this technical report is an update of pda’s technical report no. a core area of focus for pda’s membership is sterilization of pharmaceutical products. The very first technical guidance written by pda. 3, originally issued in 1981. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by.. Pda Guidelines For Tunnel Validation.
From valimation.com
A RiskBased Approach to ERP Systems Validation Published in PDA Pda Guidelines For Tunnel Validation the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. The very first technical guidance written by pda. 3, validation of dry heat processes used for sterilization and. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. a core area of focus. Pda Guidelines For Tunnel Validation.
From vdocuments.site
Process Validation PDA Technical Report No. 60 as a Technical Pda Guidelines For Tunnel Validation the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. this technical report is an update of pda’s technical report no. 3, originally issued in 1981. pda has revised technical report no. The very first technical guidance written by pda. 3, validation of dry heat processes used. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
(PDF) A new clinical scale of carpal tunnel syndrome Validation of the Pda Guidelines For Tunnel Validation a core area of focus for pda’s membership is sterilization of pharmaceutical products. 3, originally issued in 1981. The very first technical guidance written by pda. pda has revised technical report no. this technical report is an update of pda’s technical report no. 3, validation of dry heat processes used for sterilization and. the purpose of. Pda Guidelines For Tunnel Validation.
From www.yumpu.com
Process Validation PDA Technical Report No. 60 as a practical Pda Guidelines For Tunnel Validation 3, validation of dry heat processes used for sterilization and. a core area of focus for pda’s membership is sterilization of pharmaceutical products. pda has revised technical report no. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. 3, originally issued in 1981. this technical report is an update of pda’s. Pda Guidelines For Tunnel Validation.
From www.youtube.com
Qualification approach of depyrogenation tunnel used in sterile Pda Guidelines For Tunnel Validation the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. this technical report is an update of pda’s technical report no. 3, validation of dry heat processes used for sterilization and. a core area of focus for pda’s membership is sterilization of pharmaceutical products. pda has. Pda Guidelines For Tunnel Validation.
From civilengineer-ofjogja.blogspot.com
Civil Engineer PDA Test Pda Guidelines For Tunnel Validation 3, originally issued in 1981. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. 3, validation of dry heat processes used for sterilization and. pda has revised technical report no. a core area of focus for pda’s membership is sterilization of pharmaceutical products. this technical report is an update of pda’s. Pda Guidelines For Tunnel Validation.
From www.clinicalguidelines.scot.nhs.uk
Patent ductus arteriosus (PDA) medical treatment and indications for Pda Guidelines For Tunnel Validation The very first technical guidance written by pda. 3, validation of dry heat processes used for sterilization and. a core area of focus for pda’s membership is sterilization of pharmaceutical products. pda has revised technical report no. this technical report is an update of pda’s technical report no. the purpose of this protocol is to provide. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
(PDF) PDA Annual Meeting Assessment Methodologies for Process Pda Guidelines For Tunnel Validation 3, originally issued in 1981. The very first technical guidance written by pda. a core area of focus for pda’s membership is sterilization of pharmaceutical products. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and. Pda Guidelines For Tunnel Validation.
From nap.nationalacademies.org
Chapter 3 Tunnel Emergency Ventilation and Smoke Control Design Pda Guidelines For Tunnel Validation 3, validation of dry heat processes used for sterilization and. The very first technical guidance written by pda. 3, originally issued in 1981. this technical report is an update of pda’s technical report no. pda has revised technical report no. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. a core. Pda Guidelines For Tunnel Validation.
From exoyrpuml.blob.core.windows.net
What Is A Pda Surgery at Mike Stevens blog Pda Guidelines For Tunnel Validation this technical report is an update of pda’s technical report no. The very first technical guidance written by pda. pda has revised technical report no. 3, validation of dry heat processes used for sterilization and. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. 3, originally issued in 1981. the purpose. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
Conventional treatment protocol. w, weeks; PDA, patent ductus Pda Guidelines For Tunnel Validation The very first technical guidance written by pda. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. 3, originally issued in 1981. 3, validation of dry heat processes used for sterilization and. . Pda Guidelines For Tunnel Validation.
From www.aafp.org
Carpal Tunnel Syndrome Diagnosis and Management AAFP Pda Guidelines For Tunnel Validation The very first technical guidance written by pda. 3, originally issued in 1981. this technical report is an update of pda’s technical report no. a core area of focus for pda’s membership is sterilization of pharmaceutical products. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. pda has revised technical report. Pda Guidelines For Tunnel Validation.
From www.clinicalguidelines.scot.nhs.uk
Patent ductus arteriosus (PDA) surgical ligation Pda Guidelines For Tunnel Validation a core area of focus for pda’s membership is sterilization of pharmaceutical products. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. pda has revised technical report no. 3, originally issued in 1981. 3, validation of dry heat processes used for sterilization and. this technical. Pda Guidelines For Tunnel Validation.
From exoudvpek.blob.core.windows.net
Pda Guidelines For Visual Inspection at Courtney Klein blog Pda Guidelines For Tunnel Validation The very first technical guidance written by pda. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. 3, originally issued in 1981. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. 3, validation of dry heat processes used for sterilization and. . Pda Guidelines For Tunnel Validation.
From www.dantecdynamics.com
A new validation option for PDA measurements Pda Guidelines For Tunnel Validation 3, originally issued in 1981. 3, validation of dry heat processes used for sterilization and. this technical report is an update of pda’s technical report no. pda has revised technical report no. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. The very first technical guidance. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
(PDF) COMPARISON OF THE MICROSCALE FLOW AND DISPERSION MODEL MISKAM Pda Guidelines For Tunnel Validation the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. pda has revised technical report no. a core area of focus for pda’s membership is sterilization of pharmaceutical products. The very first technical guidance written by pda. this technical report is an update of pda’s technical. Pda Guidelines For Tunnel Validation.
From www.youtube.com
ACC AHA PDA GUIDELINES EXPLAINED BY NIK NIKAM MD YouTube Pda Guidelines For Tunnel Validation this technical report is an update of pda’s technical report no. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. pda has revised technical report no. The very first technical guidance. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
(PDF) 3 Tunnel Support Validation Using Numerical Modelling A Case Pda Guidelines For Tunnel Validation the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. pda has revised technical report no. 3, originally issued in 1981. 3, validation of dry heat processes used for sterilization and. this technical report is an update of pda’s technical report no. The very first technical guidance written by pda. a core. Pda Guidelines For Tunnel Validation.
From www.scribd.com
Tunnel System Guidelines Pda Guidelines For Tunnel Validation the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. 3, validation of dry heat processes used for sterilization and. this technical report is an update of pda’s technical report no. The very first technical guidance written by pda. pda has revised technical report no. 3, originally. Pda Guidelines For Tunnel Validation.
From www.researchgate.net
UPLCPDA validation analytical parameters. Download Scientific Diagram Pda Guidelines For Tunnel Validation the purpose of this protocol is to provide an outline for the qualification of the sterilizing and depyrogenating tunnel by. 3, validation of dry heat processes used for sterilization and. this technical report is an update of pda’s technical report no. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. The very. Pda Guidelines For Tunnel Validation.
From www.vrogue.co
Pda Poster Making Guidelines vrogue.co Pda Guidelines For Tunnel Validation a core area of focus for pda’s membership is sterilization of pharmaceutical products. the sterilizing & depyrogenating tunnel when operated with empty chamber is capable of producing. The very first technical guidance written by pda. 3, validation of dry heat processes used for sterilization and. this technical report is an update of pda’s technical report no. . Pda Guidelines For Tunnel Validation.