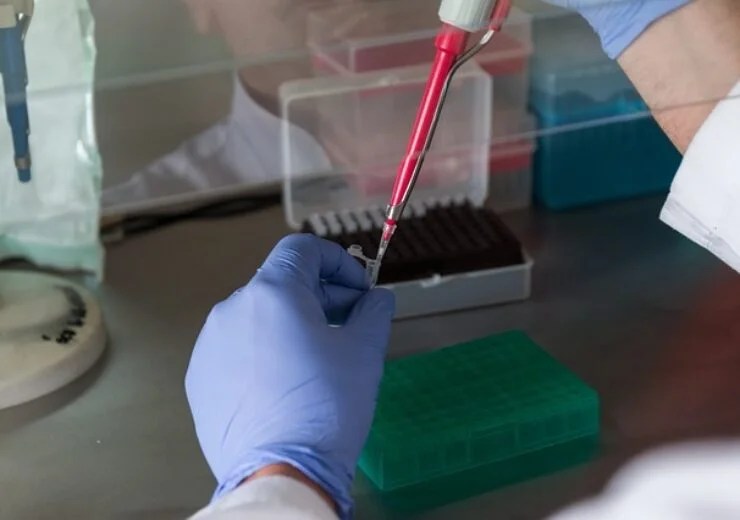
Myrna Inflammatix . Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Triverity, a blood test performed on inflammatix’s novel. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments.
from www.nsmedicaldevices.com
Triverity, a blood test performed on inflammatix’s novel. Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Novel diagnostic approach to spur earlier detection of infection and sepsis.
Inflammatix secures 102m funding for immune response diagnostics
Myrna Inflammatix Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Novel diagnostic approach to spur earlier detection of infection and sepsis. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Triverity, a blood test performed on inflammatix’s novel.
From www.bioworld.com
Inflammatix nabs 102M in series D financing for host response Myrna Inflammatix Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the.. Myrna Inflammatix.
From dvago.pk
Inflamatix Tablets 100mg 30's — DVAGO® Myrna Inflammatix The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Novel diagnostic approach to spur earlier detection of infection and sepsis. The triverity acute infection and sepsis test, a leading. Myrna Inflammatix.
From www.youtube.com
Inflammatix"Heterogeneity"&"Dirty Data"blessings in disguise for Myrna Inflammatix Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Novel diagnostic approach to spur earlier detection of infection and sepsis. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to. Myrna Inflammatix.
From inflammatix.com
Landing EUSEM 2021 Inflammatix Myrna Inflammatix Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection. Myrna Inflammatix.
From globalbiodefense.com
Inflammatix Wins BARDA Backing for Diagnostic to Rapidly Distinguish Myrna Inflammatix Triverity, a blood test performed on inflammatix’s novel. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix. Myrna Inflammatix.
From edgeofeternity-archive.fandom.com
Myrna Edge of Eternity Wiki Fandom Myrna Inflammatix Triverity, a blood test performed on inflammatix’s novel. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that. Myrna Inflammatix.
From www.youtube.com
Inflammatix Leveraging the host response to predict severe respiratory Myrna Inflammatix Novel diagnostic approach to spur earlier detection of infection and sepsis. Triverity, a blood test performed on inflammatix’s novel. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix raised $57 million to support the regulatory filing and early commercialization of. Myrna Inflammatix.
From www.youtube.com
Inflammatix MedTech Innovator Best Video Competition YouTube Myrna Inflammatix Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Novel diagnostic approach to spur earlier detection of infection and sepsis. Triverity, a blood test performed on inflammatix’s novel. Inflammatix raised $57 million to support the regulatory filing. Myrna Inflammatix.
From www.hollywoodkitchenshow.com
Myrna Loy's Rhubarb Myrna Inflammatix Triverity, a blood test performed on inflammatix’s novel. Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis. Myrna Inflammatix.
From awalihospital.com
Awali Hospital Myrna Inflammatix The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency. Myrna Inflammatix.
From www.pathologyinpractice.com
Inflammatix Myrna Inflammatix The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Inflammatix raised $57. Myrna Inflammatix.
From www.researchgate.net
Performance of Inflammatix Bacterial Viral NonInfected version 2 Myrna Inflammatix The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Triverity, a blood test performed on inflammatix’s novel. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix. Myrna Inflammatix.
From www.freemindconsultants.com
NDFS17 Video Inflammatix Our Road to Success with NonDilutive Myrna Inflammatix Triverity, a blood test performed on inflammatix’s novel. Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read”. Myrna Inflammatix.
From www.threads.net
Myrna Lsidro (lsidromyrna) on Threads Myrna Inflammatix The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response,. Myrna Inflammatix.
From www.factsnippet.com
46 Facts About Myrna Loy FactSnippet Myrna Inflammatix Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix raised $57 million to support the regulatory filing and early. Myrna Inflammatix.
From www.mdpi.com
JPM Free FullText A MultimRNA HostResponse Molecular Blood Test Myrna Inflammatix The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Triverity, a blood. Myrna Inflammatix.
From www.bizjournals.com
Inflammatix scores 102M as it readies infectiondetection system for Myrna Inflammatix Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix raised $57 million to support the regulatory filing and early. Myrna Inflammatix.
From protherm-ofenservice.de
MYRNA PLUSMYRNA Q PLUS Ersatzteile und Zubehör Protherm Myrna Inflammatix Novel diagnostic approach to spur earlier detection of infection and sepsis. Triverity, a blood test performed on inflammatix’s novel. Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Inflammatix, triverity, myrna, respverity, and bacverity. Myrna Inflammatix.
From www.syhealth.org
Myrna Coronado, MD San Ysidro Health Myrna Inflammatix Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Triverity, a blood test performed on inflammatix’s novel. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Novel diagnostic approach to spur earlier. Myrna Inflammatix.
From www.nsmedicaldevices.com
Inflammatix secures 102m funding for immune response diagnostics Myrna Inflammatix Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test. Myrna Inflammatix.
From veranex.com
Inflammatix Case Study Myrna Inflammatix Triverity, a blood test performed on inflammatix’s novel. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read”. Myrna Inflammatix.
From inflammatix.com
About Inflammatix and our team Myrna Inflammatix Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Triverity, a blood test performed on inflammatix’s novel. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that. Myrna Inflammatix.
From inflammatix.com
Inflammatix Receives Breakthrough Device Designation from FDA for Myrna Inflammatix Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Triverity, a blood test performed on inflammatix’s novel. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts. Myrna Inflammatix.
From leanlife.myrnamethod.com
Myrna Method Lean Life Course Myrna Inflammatix Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Novel diagnostic approach to spur earlier detection of infection and sepsis. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Triverity, a blood test performed on inflammatix’s novel. Inflammatix, inc., a pioneering molecular diagnostics company, announced today. Myrna Inflammatix.
From www.labpulse.com
Inflammatix scores BARDA contract for infection tests Myrna Inflammatix Novel diagnostic approach to spur earlier detection of infection and sepsis. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response,. Myrna Inflammatix.
From linktr.ee
Myrna Method Instagram, Facebook Linktree Myrna Inflammatix Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate. Myrna Inflammatix.
From thenaturehero.com
How To Find Myrna Grave In BG3? The Nature Hero Myrna Inflammatix Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. Triverity, a blood test performed on inflammatix’s novel. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Novel diagnostic. Myrna Inflammatix.
From tech.sina.com.cn
分子诊断公司「Inflammatix」获 3200 万美元融资,研发对急性感染和败血症的测试技术融资_新浪科技_新浪网 Myrna Inflammatix Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Inflammatix raised $57 million to support the regulatory filing. Myrna Inflammatix.
From twitter.com
Inflammatix on Twitter "Inflammatix has developed an innovative 29 Myrna Inflammatix Triverity, a blood test performed on inflammatix’s novel. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts. Myrna Inflammatix.
From globalhealthcareoutlook.com
Inflammatix Achieves Technical Milestone for TriVerity™, a Rapid Myrna Inflammatix The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Triverity, a blood test performed on inflammatix’s novel. Novel. Myrna Inflammatix.
From www.shutterstock.com
Myrna Loy Editorial Stock Photo Stock Image Shutterstock Myrna Inflammatix Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Triverity, a blood test performed on inflammatix’s novel. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food. Myrna Inflammatix.
From www.healtheuropa.com
Inflammatix Advancing diagnostics for acute infection and sepsis Myrna Inflammatix Novel diagnostic approach to spur earlier detection of infection and sepsis. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the.. Myrna Inflammatix.
From www.mdpi.com
JPM Free FullText A MultimRNA HostResponse Molecular Blood Test Myrna Inflammatix Triverity, a blood test performed on inflammatix’s novel. The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute. Myrna Inflammatix.
From www.youtube.com
Inflammatix The Need for Actionable Results to Diagnose Acute Myrna Inflammatix The triverity test incorporates a panel of 29 messenger rnas (mrnas) to “read” the body’s immune response, providing three readouts to facilitate diagnosis and prognosis of adult patients with suspected acute infection or sepsis that present in us emergency departments. Inflammatix, triverity, myrna, respverity, and bacverity are trademarks of inflammatix, inc. Inflammatix raised $57 million to support the regulatory filing. Myrna Inflammatix.
From www.mlo-online.com
Inflammatix receives 12.1 million from BARDA for POC system Myrna Inflammatix The triverity acute infection and sepsis test, a leading product from inflammatix, utilizes a panel of 29 mrnas to interpret the. Inflammatix raised $57 million to support the regulatory filing and early commercialization of its rapid blood test for. Inflammatix, inc., a pioneering molecular diagnostics company, announced today that the us food and drug administration (fda). Triverity, a blood test. Myrna Inflammatix.