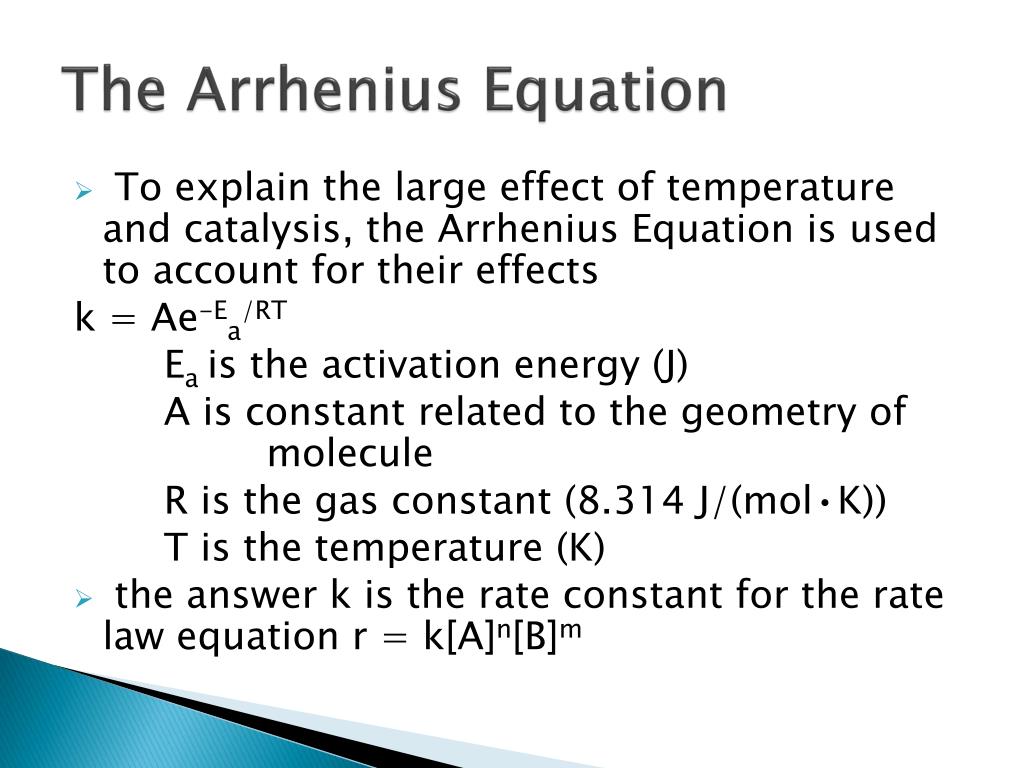
Catalyst Arrhenius Equation . The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. What the various symbols mean: If the rate constant doubles, for example,. The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. The function of a catalyst is to lower the activation energy required by a reaction. Does the arrhenius equation account for catalysts? A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. Temperature, t to fit into the equation, this has to be meaured in kelvin.
from www.slideserve.com
The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Therefore, the lowered activation energy (accounted. If the rate constant doubles, for example,. Does the arrhenius equation account for catalysts? The function of a catalyst is to lower the activation energy required by a reaction. A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. Temperature, t to fit into the equation, this has to be meaured in kelvin. What the various symbols mean: If the rate constant doubles, for example,.
PPT Reaction Mechanisms & Arrhenius Equation PowerPoint Presentation
Catalyst Arrhenius Equation Therefore, the lowered activation energy (accounted. The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Does the arrhenius equation account for catalysts? The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. If the rate constant doubles, for example,. What the various symbols mean: If the rate constant doubles, for example,. Temperature, t to fit into the equation, this has to be meaured in kelvin. Therefore, the lowered activation energy (accounted. A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. The function of a catalyst is to lower the activation energy required by a reaction.
From byjus.com
arrhenius equation Catalyst Arrhenius Equation Does the arrhenius equation account for catalysts? A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. Temperature, t to fit into the equation, this has to be meaured in kelvin. If the rate constant doubles, for example,. What the various symbols mean: The arrhenius equation can be used. Catalyst Arrhenius Equation.
From www.youtube.com
CHEM1312_CH11.67 Arrhenius Equation and catalyst_part 1 YouTube Catalyst Arrhenius Equation If the rate constant doubles, for example,. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. What the various symbols mean: Does the arrhenius equation account for catalysts? The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant,. Catalyst Arrhenius Equation.
From solvedlib.com
Apply the Arrhenius equation to calculate how many ti… SolvedLib Catalyst Arrhenius Equation A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. Temperature, t to fit into the equation, this has to be meaured in kelvin. The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. If the rate constant doubles, for example,. Does the arrhenius equation account for catalysts? The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. If the rate constant doubles, for example,. What the various. Catalyst Arrhenius Equation.
From www.geeksforgeeks.org
Arrhenius Equation Explanation, Graph, and Solved Examples Catalyst Arrhenius Equation What the various symbols mean: The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. Does the arrhenius equation account for catalysts? The function of a catalyst is to lower the activation energy required by a reaction. If the rate constant doubles, for example,. Therefore, the lowered activation. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. The function of a catalyst is to lower the activation energy required by a reaction. Does the arrhenius equation account for catalysts? A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. What the various. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Catalyst Arrhenius Equation The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Therefore, the lowered activation energy (accounted. If the rate constant doubles, for example,. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a. Catalyst Arrhenius Equation.
From www.slideshare.net
D08 abbrev arrhenius and catalysts_alg Catalyst Arrhenius Equation The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. If the rate constant doubles, for example,. The function of a catalyst is to lower the activation energy required by. Catalyst Arrhenius Equation.
From www.tes.com
The Arrhenius Equation (A Level Chemistry) Teaching Resources Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. What the various symbols mean: The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. If the rate constant doubles, for example,. The equation shows that either increasing the. Catalyst Arrhenius Equation.
From chemistnotes.com
Two point arrhenius equation, Easy derivation, 3 application Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. If the rate constant doubles, for example,. If the rate constant doubles, for example,. The function of a catalyst is to lower the activation energy required by a reaction. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the. Catalyst Arrhenius Equation.
From wiringfixunripping.z21.web.core.windows.net
Reaction Energy Diagram With Catalyst Catalyst Arrhenius Equation If the rate constant doubles, for example,. Does the arrhenius equation account for catalysts? The function of a catalyst is to lower the activation energy required by a reaction. If the rate constant doubles, for example,. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. The arrhenius. Catalyst Arrhenius Equation.
From www.youtube.com
CHEM1312_CH11.67 Arrhenius Equation and catalyst_part 2 YouTube Catalyst Arrhenius Equation Therefore, the lowered activation energy (accounted. The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Does the arrhenius equation account for catalysts? If the rate constant doubles, for example,. If the rate constant doubles, for example,. What the various symbols mean: The. Catalyst Arrhenius Equation.
From www.researchgate.net
arrhenius plot of catalytic data and apparent activation energies Catalyst Arrhenius Equation What the various symbols mean: The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. If the rate constant doubles, for example,. Temperature, t to fit into the equation, this has to be meaured in kelvin. The function of a catalyst is to lower the activation energy required. Catalyst Arrhenius Equation.
From www.youtube.com
Activation energy/Arrhenius equation/Effect of catalyst and Temperature Catalyst Arrhenius Equation If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. Does the arrhenius equation account for catalysts? The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. If the rate constant doubles, for example,. What the various symbols mean: A combination of equations 1 and. Catalyst Arrhenius Equation.
From rumble.com
Arrhenius Equation, Chemistry Catalyst Arrhenius Equation The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. What the various symbols mean: Temperature, t to fit into the equation, this has to be meaured in kelvin. Does. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT The Arrhenius Equation PowerPoint Presentation, free download Catalyst Arrhenius Equation The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. What the various symbols mean: Does the arrhenius equation account for catalysts? Temperature, t to fit into the equation, this has to be meaured in kelvin. If the rate constant doubles, for example,. If the rate constant doubles,. Catalyst Arrhenius Equation.
From www.toppr.com
In the Arrhenius equation, k = Ae^Ea/RT , the Arrhenius constant A Catalyst Arrhenius Equation Therefore, the lowered activation energy (accounted. What the various symbols mean: Does the arrhenius equation account for catalysts? If the rate constant doubles, for example,. The function of a catalyst is to lower the activation energy required by a reaction. If the rate constant doubles, for example,. A combination of equations 1 and 2 gives the arrhenius equation, which shows. Catalyst Arrhenius Equation.
From www.researchgate.net
Linearization plot of the Arrhenius equation to estimate the Catalyst Arrhenius Equation What the various symbols mean: Does the arrhenius equation account for catalysts? A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. If the rate constant doubles, for example,. Temperature, t to fit into the equation, this has to be meaured in kelvin. The equation shows that either increasing. Catalyst Arrhenius Equation.
From www.dreamstime.com
Arrhenius Equation Physical Chemistry Science Vector Infographic Stock Catalyst Arrhenius Equation Does the arrhenius equation account for catalysts? The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. What the various symbols mean: The function of a catalyst is to lower the activation energy required by a reaction. If the rate constant doubles, for example,. Therefore, the lowered activation. Catalyst Arrhenius Equation.
From www.slideshare.net
Chem 2 Chemical VIII The Arrhenius Equation, Activation E… Catalyst Arrhenius Equation The function of a catalyst is to lower the activation energy required by a reaction. If the rate constant doubles, for example,. The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Does the arrhenius equation account for catalysts? Therefore, the lowered activation. Catalyst Arrhenius Equation.
From www.slideshare.net
D07 abbrev arrhenius and catalysts_alg Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. Therefore, the lowered activation energy (accounted. Does the arrhenius equation account for catalysts? The arrhenius equation can be used to determine the effect of a. Catalyst Arrhenius Equation.
From www.studypool.com
SOLUTION The arrhenius equation Studypool Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. The function of a catalyst is to lower the activation energy required by a reaction. What the various symbols mean: Therefore, the lowered activation. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT Catalyst Fundamentals PowerPoint Presentation, free download ID Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. If the rate constant doubles, for example,. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. The arrhenius equation can. Catalyst Arrhenius Equation.
From atomstalk.com
Arrhenius Equation AtomsTalk Catalyst Arrhenius Equation Does the arrhenius equation account for catalysts? The function of a catalyst is to lower the activation energy required by a reaction. If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. A combination of. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT Arrhenius equation PowerPoint Presentation, free download ID Catalyst Arrhenius Equation What the various symbols mean: The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Temperature, t to fit into the equation, this has to be meaured in kelvin. Therefore, the lowered activation energy (accounted. A combination of equations 1 and 2 gives. Catalyst Arrhenius Equation.
From www.youtube.com
The Arrhenius Equation, Activation Energy, and Catalysis Explained Pt 8 Catalyst Arrhenius Equation If the rate constant doubles, for example,. The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. Temperature, t to fit into the equation, this has to be meaured in kelvin. The equation shows that either increasing the temperature or decreasing the activation. Catalyst Arrhenius Equation.
From www.numerade.com
SOLVEDA catalyst lowers the activation energy of a reaction from 215 Catalyst Arrhenius Equation Does the arrhenius equation account for catalysts? The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. A combination of equations. Catalyst Arrhenius Equation.
From ecuacionde.com
Ecuación de Arrhenius Teoría, ejemplos y más Catalyst Arrhenius Equation If the rate constant doubles, for example,. The function of a catalyst is to lower the activation energy required by a reaction. What the various symbols mean: If the rate constant doubles, for example,. Temperature, t to fit into the equation, this has to be meaured in kelvin. Therefore, the lowered activation energy (accounted. Does the arrhenius equation account for. Catalyst Arrhenius Equation.
From study.com
Using the Arrhenius Equation Chemistry Catalyst Arrhenius Equation The function of a catalyst is to lower the activation energy required by a reaction. If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. If the rate constant doubles, for example,. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. Does the arrhenius. Catalyst Arrhenius Equation.
From byjus.com
54 In the presence of a catalyst activation energy of a reaction is Catalyst Arrhenius Equation A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. The function of a catalyst is to lower the activation energy required by a reaction. Does the. Catalyst Arrhenius Equation.
From demonstrations.wolfram.com
Simple Arrhenius Model for Activation Energy and Catalysis Wolfram Catalyst Arrhenius Equation Temperature, t to fit into the equation, this has to be meaured in kelvin. The function of a catalyst is to lower the activation energy required by a reaction. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. The arrhenius equation can be used to determine the. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT Chemical PowerPoint Presentation, free download ID409487 Catalyst Arrhenius Equation What the various symbols mean: A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. The arrhenius equation can be used to determine the effect of a change of temperature on the rate constant, and consequently on the rate of the reaction. The equation shows that either increasing the. Catalyst Arrhenius Equation.
From www.youtube.com
6 Chemical Collision Theory Arrhenius Equation Catalyst Arrhenius Equation If the rate constant doubles, for example,. Therefore, the lowered activation energy (accounted. Does the arrhenius equation account for catalysts? If the rate constant doubles, for example,. What the various symbols mean: The function of a catalyst is to lower the activation energy required by a reaction. Temperature, t to fit into the equation, this has to be meaured in. Catalyst Arrhenius Equation.
From www.slideserve.com
PPT Reaction Mechanisms & Arrhenius Equation PowerPoint Presentation Catalyst Arrhenius Equation Therefore, the lowered activation energy (accounted. What the various symbols mean: The function of a catalyst is to lower the activation energy required by a reaction. The equation shows that either increasing the temperature or decreasing the activation energy (for example through the use of a catalyst) will. Temperature, t to fit into the equation, this has to be meaured. Catalyst Arrhenius Equation.
From goosys.weebly.com
Arrhenius Equation Activation Energy Derivation Definition goosys Catalyst Arrhenius Equation The function of a catalyst is to lower the activation energy required by a reaction. A combination of equations 1 and 2 gives the arrhenius equation, which shows how the rate constant is dependent on temperature. If the rate constant doubles, for example,. What the various symbols mean: The arrhenius equation can be used to determine the effect of a. Catalyst Arrhenius Equation.