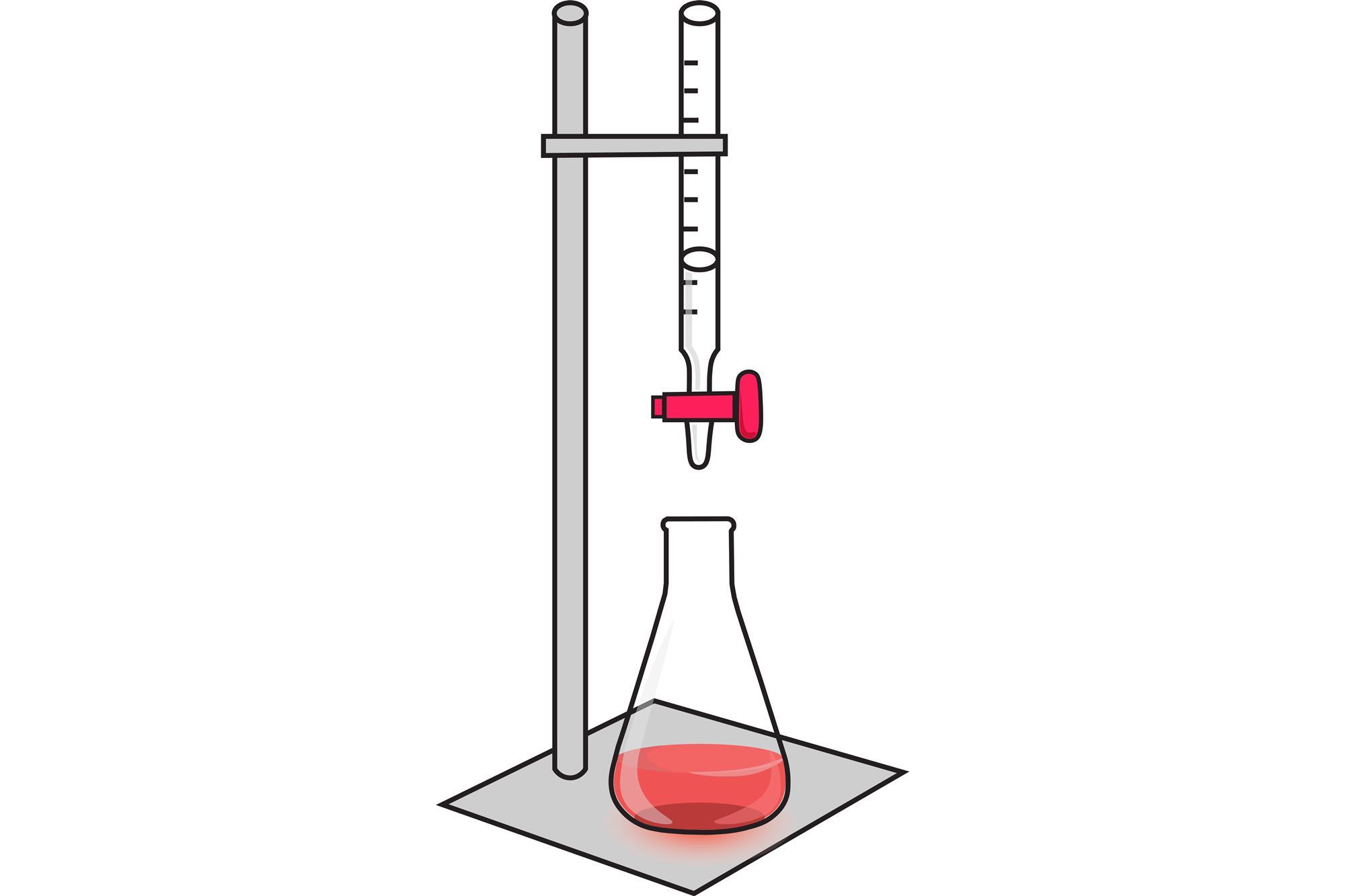
Titration Experiment Evaluation . The alkali usually in conical. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. The steps in a titration. The titration of a weak acid with a strong base will be examined theoretically in this section. The calculations will then be used to plot a titration. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. They then concentrate the solution and allow it to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Bring together organic chemistry and moles calculations with the aspirin screen experiment. Some general calculations you may need to complete to evaluate an experimental procedure: A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour.
from quizizz.com
Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. Bring together organic chemistry and moles calculations with the aspirin screen experiment. Some general calculations you may need to complete to evaluate an experimental procedure: The titration of a weak acid with a strong base will be examined theoretically in this section. The calculations will then be used to plot a titration. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. They then concentrate the solution and allow it to. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The steps in a titration. The alkali usually in conical.
Titration Method 186 plays Quizizz
Titration Experiment Evaluation They then concentrate the solution and allow it to. The calculations will then be used to plot a titration. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. Bring together organic chemistry and moles calculations with the aspirin screen experiment. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. Some general calculations you may need to complete to evaluate an experimental procedure: The alkali usually in conical. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. The steps in a titration. The titration of a weak acid with a strong base will be examined theoretically in this section.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Experiment Evaluation The calculations will then be used to plot a titration. The titration of a weak acid with a strong base will be examined theoretically in this section. The alkali usually in conical. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. They then concentrate the solution and allow. Titration Experiment Evaluation.
From www.chegg.com
Solved Lab Report Evaluating Antacids via a Titration Titration Experiment Evaluation Bring together organic chemistry and moles calculations with the aspirin screen experiment. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. The steps in a titration. A white tile is placed under the conical flask while the titration is performed, to make it easier. Titration Experiment Evaluation.
From ar.inspiredpencil.com
Titration Diagram Titration Experiment Evaluation The steps in a titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Bring together organic chemistry and moles calculations with the aspirin screen experiment. Some general calculations you may need to complete to evaluate an experimental procedure: The calculations will then be used to plot a titration. A. Titration Experiment Evaluation.
From quizizz.com
Titration Method 186 plays Quizizz Titration Experiment Evaluation The alkali usually in conical. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. A white tile is placed under the conical flask while the. Titration Experiment Evaluation.
From pixels.com
Titration Experiment Photograph by Titration Experiment Evaluation The alkali usually in conical. The titration of a weak acid with a strong base will be examined theoretically in this section. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. If solution a is titrated against solution b, it means that solution a is in the conical. Titration Experiment Evaluation.
From www.studocu.com
Experiment 2 Acid Base Titration Evaluation Question 1 10/10/23, 12 Titration Experiment Evaluation The alkali usually in conical. The titration of a weak acid with a strong base will be examined theoretically in this section. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. Bring together organic chemistry and moles calculations with the aspirin screen experiment. The. Titration Experiment Evaluation.
From www.youtube.com
️ Titration Experiment for Board Class Complete Video to Understand Titration Experiment Evaluation Bring together organic chemistry and moles calculations with the aspirin screen experiment. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. The steps in a titration. The alkali usually in conical. Some general calculations you may need to complete to evaluate an experimental procedure: They then concentrate the. Titration Experiment Evaluation.
From themasterchemistry.com
Acid Base TitrationWorking Principle, Process, Types And Indicators Titration Experiment Evaluation Bring together organic chemistry and moles calculations with the aspirin screen experiment. The titration of a weak acid with a strong base will be examined theoretically in this section. The steps in a titration. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. A white tile is placed. Titration Experiment Evaluation.
From www.sciencephoto.com
Titration experiment Stock Image C004/7692 Science Photo Library Titration Experiment Evaluation In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to. The steps in a titration. The titration of a weak acid with a strong base will be examined theoretically in this section. Some general calculations you may need to complete to evaluate. Titration Experiment Evaluation.
From chem4three.blogspot.ca
CHEMISTRY 11 TITRATIONS Titration Experiment Evaluation The steps in a titration. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. Some general calculations you may need to complete to evaluate an experimental procedure: A white tile is placed under the conical flask while the titration is performed, to make it easier to see the. Titration Experiment Evaluation.
From www.studocu.com
CHEM 3401 Amino Acid Titration Experiment FA21 Amino Acid Titration Titration Experiment Evaluation Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. They then concentrate the solution and allow it to. Some general calculations you may need to complete to evaluate an experimental procedure: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride. Titration Experiment Evaluation.
From www.studocu.com
Experiment two Titration EXPERIMENT 2 STANDARDIZATION OF HCl Titration Experiment Evaluation Bring together organic chemistry and moles calculations with the aspirin screen experiment. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titrations are done often to find out. Titration Experiment Evaluation.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Experiment Evaluation Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. The calculations will then be used to plot a titration. They then concentrate the solution and allow it to. The alkali usually in conical. Bring together organic chemistry and moles calculations with the aspirin screen experiment. The steps in. Titration Experiment Evaluation.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Titration Experiment Evaluation The alkali usually in conical. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. If solution a is titrated against solution b, it means that solution a is. Titration Experiment Evaluation.
From chemistnotes.com
Precipitation Titration Principle, Types, and 5 Reliable Applications Titration Experiment Evaluation A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. The calculations will then be used to plot a titration. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. The steps in a titration. In this. Titration Experiment Evaluation.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titration Experiment Evaluation Some general calculations you may need to complete to evaluate an experimental procedure: They then concentrate the solution and allow it to. The titration of a weak acid with a strong base will be examined theoretically in this section. The steps in a titration. If solution a is titrated against solution b, it means that solution a is in the. Titration Experiment Evaluation.
From www.tes.com
How to teach titrations for GCSE science Tes Titration Experiment Evaluation Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. They then concentrate the solution and allow it to. The alkali usually in conical. Some general calculations you may need to complete to evaluate an experimental procedure: The calculations will then be used to plot a titration. Bring together. Titration Experiment Evaluation.
From fineartamerica.com
Titration Experiment Photograph by Titration Experiment Evaluation A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. They then concentrate the solution and allow it to. The alkali usually in conical. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the. Titration Experiment Evaluation.
From www.slideshare.net
Titration Lab Report Titration Experiment Evaluation The alkali usually in conical. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The calculations will then be used to plot a titration. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. They then concentrate the solution. Titration Experiment Evaluation.
From askfilo.com
In a titration experiment where NH4 OH is kept in the titration flask and.. Titration Experiment Evaluation The calculations will then be used to plot a titration. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. The titration of a weak acid with a strong base will be examined theoretically in this section. They then concentrate the solution and allow it to. In this experiment. Titration Experiment Evaluation.
From www.dreamstime.com
Titration Technique in the Laboratory. Stock Photo Image of fluid Titration Experiment Evaluation They then concentrate the solution and allow it to. Bring together organic chemistry and moles calculations with the aspirin screen experiment. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. A white tile is placed under the conical flask while the titration is performed,. Titration Experiment Evaluation.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration Experiment Evaluation The titration of a weak acid with a strong base will be examined theoretically in this section. The alkali usually in conical. The calculations will then be used to plot a titration. Some general calculations you may need to complete to evaluate an experimental procedure: In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt. Titration Experiment Evaluation.
From www.studocu.com
20300492 Experiment 3 Acid and Base Titration EXPERIMENT 3 ACID AND Titration Experiment Evaluation Bring together organic chemistry and moles calculations with the aspirin screen experiment. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. The steps in a titration. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is. Titration Experiment Evaluation.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Experiment Evaluation The titration of a weak acid with a strong base will be examined theoretically in this section. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. Bring together organic chemistry and moles calculations with the aspirin screen experiment. Titrations are done often to find. Titration Experiment Evaluation.
From mavink.com
Titration Labeled Titration Experiment Evaluation Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. If solution a is titrated against solution b, it means that solution a is in the conical. Titration Experiment Evaluation.
From www.savemyexams.co.uk
Titrations (1.7.2) Edexcel A Level Chemistry Revision Notes 2017 Titration Experiment Evaluation The titration of a weak acid with a strong base will be examined theoretically in this section. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. A white tile is placed under the conical flask while the titration is performed, to make it easier. Titration Experiment Evaluation.
From www.youtube.com
Titration Experiment chemistry practical experiment Titration Experiment Evaluation The alkali usually in conical. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. Bring together organic chemistry and moles calculations with the aspirin screen. Titration Experiment Evaluation.
From www.reagent.co.uk
Who Invented Titration? The Science Blog Titration Experiment Evaluation Bring together organic chemistry and moles calculations with the aspirin screen experiment. The titration of a weak acid with a strong base will be examined theoretically in this section. The steps in a titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Some general calculations you may need to. Titration Experiment Evaluation.
From mavink.com
Titration Labeled Titration Experiment Evaluation In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. They then concentrate the solution and allow it to. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. The alkali usually in conical. If solution a is titrated. Titration Experiment Evaluation.
From mungfali.com
Acid Base Titration Calculation Titration Experiment Evaluation A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. The alkali usually in conical. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The calculations will then be used to plot a titration. They then concentrate the. Titration Experiment Evaluation.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom Titration Experiment Evaluation They then concentrate the solution and allow it to. The alkali usually in conical. The calculations will then be used to plot a titration. The steps in a titration. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. The titration of a weak acid with a strong. Titration Experiment Evaluation.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Titration Experiment Evaluation The titration of a weak acid with a strong base will be examined theoretically in this section. Titrations are done often to find out the concentration of one substance by reacting it with another substance of known concentration. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour.. Titration Experiment Evaluation.
From www.rdworldonline.com
What are titration instruments? Research & Development World Titration Experiment Evaluation Bring together organic chemistry and moles calculations with the aspirin screen experiment. A white tile is placed under the conical flask while the titration is performed, to make it easier to see the colour. The calculations will then be used to plot a titration. The titration of a weak acid with a strong base will be examined theoretically in this. Titration Experiment Evaluation.
From www.sciencephoto.com
Titration experiment Stock Image C010/9616 Science Photo Library Titration Experiment Evaluation The calculations will then be used to plot a titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The steps in a titration. The alkali usually in conical. Some general calculations you may need to complete to evaluate an experimental procedure: A white tile is placed under the conical. Titration Experiment Evaluation.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Experiment Evaluation If solution a is titrated against solution b, it means that solution a is in the conical flask and solution b is in the burette. The calculations will then be used to plot a titration. In this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Bring together organic chemistry and moles. Titration Experiment Evaluation.