What Is A Catalyst And How Does It Work . In heterogeneous catalysis, catalysts provide a surface to. Describe the similarities and differences between the three principal classes of catalysts. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts work by providing an alternative. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. A catalyst that is in a separate phase from the reactants is. Catalysis is the process of speeding up a reaction using a catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is some material that speeds up chemical reactions.
from www.autopadre.com
Enzymes are naturally occurring catalysts responsible for many. In heterogeneous catalysis, catalysts provide a surface to. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. A catalyst is some material that speeds up chemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is the process of speeding up a reaction using a catalyst. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal classes of catalysts. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed.
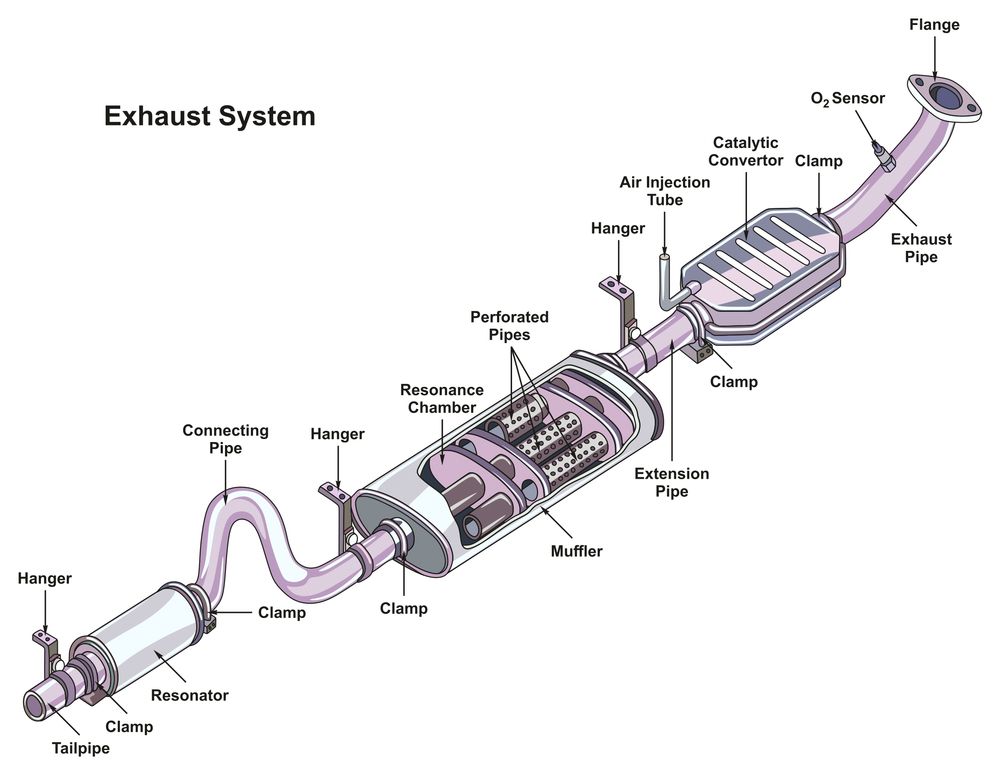
Catalytic Converter Location And Understanding Its Importance in Your
What Is A Catalyst And How Does It Work Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Describe the similarities and differences between the three principal classes of catalysts. A catalyst is some material that speeds up chemical reactions. In heterogeneous catalysis, catalysts provide a surface to. A catalyst that is in a separate phase from the reactants is. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts work by providing an alternative. Catalysis is the process of speeding up a reaction using a catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst.
From www.waca.msf.org
Lesson Explainer Catalysts, Catalyst What Is A Catalyst And How Does It Work Enzymes are naturally occurring catalysts responsible for many. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst that is in a separate phase from the reactants is. Catalysts work by providing an alternative. In heterogeneous catalysis, catalysts provide a surface to. The word “catalyst” comes from. What Is A Catalyst And How Does It Work.
From www.thoughtco.com
Catalysis Definition in Chemistry What Is A Catalyst And How Does It Work A catalyst is some material that speeds up chemical reactions. Describe the similarities and differences between the three principal classes of catalysts. In heterogeneous catalysis, catalysts provide a surface to. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst in the same phase (usually liquid or gas solution) as the reactants. What Is A Catalyst And How Does It Work.
From www.tersusdef.com
Selective Catalytic Reduction How it works Tersus Diesel Exhaust What Is A Catalyst And How Does It Work Catalysts work by providing an alternative. In heterogeneous catalysis, catalysts provide a surface to. Describe the similarities and differences between the three principal classes of catalysts. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst that is in a separate phase from the reactants is. Enzymes. What Is A Catalyst And How Does It Work.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii What Is A Catalyst And How Does It Work A catalyst that is in a separate phase from the reactants is. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process.. What Is A Catalyst And How Does It Work.
From www.nettinc.com
What Is a Diesel Oxidation Catalyst? Nett Technologies What Is A Catalyst And How Does It Work In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts work by providing an alternative. Describe the similarities and differences between the three principal classes of catalysts. With a helping hand from a catalyst, molecules that. What Is A Catalyst And How Does It Work.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 What Is A Catalyst And How Does It Work Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What are catalysts, and how do they work in terms altering the parameters of a reaction? In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring. What Is A Catalyst And How Does It Work.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) What Is A Catalyst And How Does It Work With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysts work by providing an alternative. A catalyst is some material that speeds up chemical reactions. What are catalysts, and how do they work in. What Is A Catalyst And How Does It Work.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 What Is A Catalyst And How Does It Work In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. In heterogeneous catalysis, catalysts provide a surface to. Enzymes are naturally occurring catalysts responsible for many. A catalyst is some material that speeds up chemical reactions. Catalysis is the process of speeding up a reaction using a catalyst. Catalysts. What Is A Catalyst And How Does It Work.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst What Is A Catalyst And How Does It Work Enzymes are naturally occurring catalysts responsible for many. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. Catalysts allow a reaction to proceed via a pathway that has a. What Is A Catalyst And How Does It Work.
From scitechdaily.com
Science Made Simple What Are Catalysts? What Is A Catalyst And How Does It Work A catalyst is some material that speeds up chemical reactions. Describe the similarities and differences between the three principal classes of catalysts. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via. What Is A Catalyst And How Does It Work.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis What Is A Catalyst And How Does It Work Catalysts work by providing an alternative. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst that is in a separate phase from the reactants is. Catalysis is the process of speeding up a reaction using a catalyst. With a helping hand from a catalyst, molecules that. What Is A Catalyst And How Does It Work.
From engineeringlearner.com
What is Catalytic Converter? Engineering Learner What Is A Catalyst And How Does It Work Enzymes are naturally occurring catalysts responsible for many. Describe the similarities and differences between the three principal classes of catalysts. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts work by providing an alternative. In heterogeneous catalysis, catalysts provide a surface to. A catalyst in the same phase (usually liquid or gas. What Is A Catalyst And How Does It Work.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube What Is A Catalyst And How Does It Work With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Enzymes are naturally occurring catalysts responsible for many. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is some material. What Is A Catalyst And How Does It Work.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of What Is A Catalyst And How Does It Work The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface to. Catalysts work by providing an alternative. With a helping. What Is A Catalyst And How Does It Work.
From blog.olx.com.pk
Everything You Need to Know About Catalytic Converters What Is A Catalyst And How Does It Work Catalysts work by providing an alternative. Enzymes are naturally occurring catalysts responsible for many. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is some material that speeds up chemical reactions. The word “catalyst” comes from the greek word. What Is A Catalyst And How Does It Work.
From www.youtube.com
How does a CATALYST work ? YouTube What Is A Catalyst And How Does It Work With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal classes of catalysts. In chemistry, a catalyst is a substance that increases the rate of. What Is A Catalyst And How Does It Work.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of What Is A Catalyst And How Does It Work Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. With a helping hand from a catalyst, molecules that. What Is A Catalyst And How Does It Work.
From azurechemical.com
How Does a Selective Catalytic Reduction System Work? What Is A Catalyst And How Does It Work Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Describe the similarities and differences between the three principal classes of catalysts. A catalyst is some material. What Is A Catalyst And How Does It Work.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of What Is A Catalyst And How Does It Work In heterogeneous catalysis, catalysts provide a surface to. With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst that is in a separate phase from the reactants is. A catalyst in the. What Is A Catalyst And How Does It Work.
From www.fastreplacementglass.com
What Is A Catalytic Combustor & How Does It Work? What Is A Catalyst And How Does It Work In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Describe the similarities and differences between the three principal classes of catalysts. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. What are catalysts, and how do they. What Is A Catalyst And How Does It Work.
From www.slideshare.net
Catalyst What Is A Catalyst And How Does It Work In heterogeneous catalysis, catalysts provide a surface to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysis is the process of speeding up a reaction using a catalyst. Enzymes are naturally occurring catalysts responsible for many. Describe the similarities and differences between the three principal classes of catalysts. A. What Is A Catalyst And How Does It Work.
From www.catalystseurope.org
What are catalysts? What Is A Catalyst And How Does It Work Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a reaction using a catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Enzymes are naturally occurring catalysts responsible for many. Describe the similarities. What Is A Catalyst And How Does It Work.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts What Is A Catalyst And How Does It Work What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysis is the process of speeding up a reaction using a catalyst. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Describe the similarities and differences between the three principal classes of catalysts. In. What Is A Catalyst And How Does It Work.
From 2012books.lardbucket.org
Catalysis What Is A Catalyst And How Does It Work The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal classes of catalysts. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is. What Is A Catalyst And How Does It Work.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube What Is A Catalyst And How Does It Work Catalysts work by providing an alternative. Describe the similarities and differences between the three principal classes of catalysts. A catalyst is some material that speeds up chemical reactions. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Enzymes are naturally occurring catalysts responsible for many. A catalyst in the same. What Is A Catalyst And How Does It Work.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram What Is A Catalyst And How Does It Work A catalyst that is in a separate phase from the reactants is. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. The word “catalyst” comes from the greek. What Is A Catalyst And How Does It Work.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica What Is A Catalyst And How Does It Work Enzymes are naturally occurring catalysts responsible for many. In heterogeneous catalysis, catalysts provide a surface to. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst in the. What Is A Catalyst And How Does It Work.
From autotrends.org
The Function and Purpose of a Catalytic Converter Auto Trends Magazine What Is A Catalyst And How Does It Work Catalysis is the process of speeding up a reaction using a catalyst. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. What are catalysts, and how do they work in terms altering the parameters. What Is A Catalyst And How Does It Work.
From www.slideserve.com
PPT Nanocatalyst PowerPoint Presentation ID676158 What Is A Catalyst And How Does It Work What are catalysts, and how do they work in terms altering the parameters of a reaction? Describe the similarities and differences between the three principal classes of catalysts. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. Catalysts allow a reaction to proceed via a pathway that has a. What Is A Catalyst And How Does It Work.
From diagrampartlacquering.z19.web.core.windows.net
Parts Of A Catalytic Converter What Is A Catalyst And How Does It Work A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. What are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst is. What Is A Catalyst And How Does It Work.
From www.autopadre.com
Catalytic Converter Location And Understanding Its Importance in Your What Is A Catalyst And How Does It Work Catalysis is the process of speeding up a reaction using a catalyst. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. A catalyst in the same phase (usually liquid or gas solution) as the reactants and products is called homogeneous catalyst. Catalysts work by providing an alternative. Describe. What Is A Catalyst And How Does It Work.
From firecatcombustors.blogspot.com
woodstove catalytic combustors How a catalytic combustor works. What Is A Catalyst And How Does It Work Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Describe the similarities and differences between the three principal classes of catalysts. A catalyst is some material that speeds up chemical reactions. Enzymes are naturally occurring. What Is A Catalyst And How Does It Work.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B What Is A Catalyst And How Does It Work With a helping hand from a catalyst, molecules that might take years to interact can now do so in seconds. Catalysts work by providing an alternative. In heterogeneous catalysis, catalysts provide a surface to. What are catalysts, and how do they work in terms altering the parameters of a reaction? Catalysts allow a reaction to proceed via a pathway that. What Is A Catalyst And How Does It Work.
From wou.edu
Chapter 7 Catalytic Mechanisms of Enzymes Chemistry What Is A Catalyst And How Does It Work The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Enzymes are naturally occurring catalysts responsible for many. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts work by providing an alternative. Describe the similarities and differences between the three principal classes of catalysts. With a helping. What Is A Catalyst And How Does It Work.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint What Is A Catalyst And How Does It Work In heterogeneous catalysis, catalysts provide a surface to. In chemistry, a catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy. What Is A Catalyst And How Does It Work.