Calorimetry Post Lab Questions . Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Study with quizlet and memorize flashcards containing terms like part. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Study with quizlet and memorize flashcards containing terms like part a.1. The study of heat energy, temperature, and heat transfer. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Specific heat of metal mass of water This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Study with quizlet and memorize flashcards containing terms like carbohydrates,.
from studylib.net
Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Specific heat of metal mass of water Study with quizlet and memorize flashcards containing terms like part. This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like part a.1. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water.
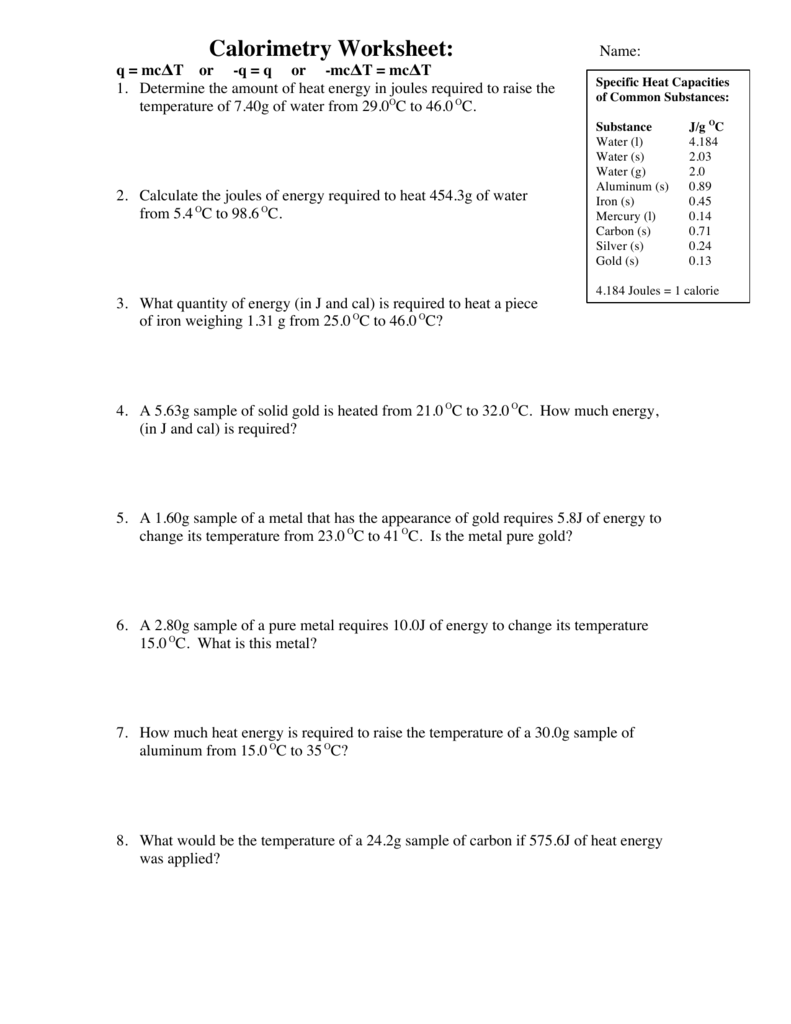
Calorimetry Worksheet
Calorimetry Post Lab Questions Specific heat of metal mass of water Study with quizlet and memorize flashcards containing terms like part. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Specific heat of metal mass of water This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. The study of heat energy, temperature, and heat transfer. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Study with quizlet and memorize flashcards containing terms like part a.1. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water.
From www.studocu.com
Calorimetry postlab Calorimetry Postlab Submission Guide Directly Calorimetry Post Lab Questions The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like carbohydrates,. Study with quizlet and memorize flashcards containing terms like part. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Abstract the main purpose. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Calorimetry Lab BEFORE YOU BEGIN post your Calorimetry Post Lab Questions Specific heat of metal mass of water Study with quizlet and memorize flashcards containing terms like part. Study with quizlet and memorize flashcards containing terms like carbohydrates,. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Therefore, the enthalpy of the solution, ∆hs, would be reported as too. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Lab 6 Calorimetry PostLab Assignment Name A Calorimetry Post Lab Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Study with quizlet and memorize flashcards containing terms like part. Study with quizlet and memorize flashcards containing terms like part a.1. The study of heat energy, temperature, and heat transfer. Therefore, the enthalpy. Calorimetry Post Lab Questions.
From www.chegg.com
Solved CHM 2045 Hear Effects and Calorimetry PostLab Calorimetry Post Lab Questions Specific heat of metal mass of water Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Study with quizlet and memorize flashcards containing terms like part. The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set. Calorimetry Post Lab Questions.
From studylib.net
Calorimetry Worksheet Calorimetry Post Lab Questions This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Abstract the main purpose. Calorimetry Post Lab Questions.
From www.chegg.com
Section Name EXPERIMENT 8 CALORIMETRY Postlab Calorimetry Post Lab Questions Study with quizlet and memorize flashcards containing terms like part. The study of heat energy, temperature, and heat transfer. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Question. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Constant Pressure CalorimetryVirtual Lab PostLab Calorimetry Post Lab Questions Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding. Calorimetry Post Lab Questions.
From www.chegg.com
Solved COFFEE CUP CALORIMETRY POSTLAB ASSIGNMENT LABORATORY Calorimetry Post Lab Questions Study with quizlet and memorize flashcards containing terms like carbohydrates,. Study with quizlet and memorize flashcards containing terms like part a.1. Specific heat of metal mass of water Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. The study of heat energy, temperature, and heat transfer. Abstract the main purpose of the calorimetry experiment is to. Calorimetry Post Lab Questions.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Post Lab Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Study with quizlet and memorize flashcards containing terms like part. This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Question 1 the energy. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Calorimetry Post Lab Questions To receive full Calorimetry Post Lab Questions Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Study with quizlet and memorize flashcards containing terms like part. The study of heat energy, temperature,. Calorimetry Post Lab Questions.
From www.chegg.com
Solved 1402 Exp 10 Calorimetry Virtual Lab PostLab Calorimetry Post Lab Questions This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Study with quizlet and memorize flashcards containing terms like carbohydrates,. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g. Calorimetry Post Lab Questions.
From www.chegg.com
Solved PostLab Questions 1. What does the calorimeter Calorimetry Post Lab Questions Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Study with quizlet and memorize flashcards containing terms like part a.1. The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the main types of questions on calorimetry. Calorimetry Post Lab Questions.
From www.chegg.com
Solved COFFEE CUP CALORIMETRY POSTLAB ASSIGNMENT LABORATORY Calorimetry Post Lab Questions Study with quizlet and memorize flashcards containing terms like part. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Study with quizlet and memorize flashcards containing terms like part. Calorimetry Post Lab Questions.
From www.chegg.com
1402 Exp 10 Calorimetry Section Virtual Lab PostLab Calorimetry Post Lab Questions Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Specific heat of metal mass of water Therefore,. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Raw Data from calorimetry analysis of Cheeto samples Calorimetry Post Lab Questions Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Specific heat of metal mass of water Study with quizlet and memorize flashcards containing terms like carbohydrates,. Study with quizlet. Calorimetry Post Lab Questions.
From studyzonejunker.z19.web.core.windows.net
Calorimetry Questions And Answers Calorimetry Post Lab Questions Study with quizlet and memorize flashcards containing terms like carbohydrates,. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. Study with quizlet and memorize flashcards containing terms like part. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat. Calorimetry Post Lab Questions.
From www.chegg.com
CALORIMETRY Postlab Questions Date Name Partners) 1. Calorimetry Post Lab Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. This is the post lab for experiment 25 which was the calorimetry. Calorimetry Post Lab Questions.
From www.coursehero.com
[Solved] CHM 111 Calorimetry Lab 2021 solve the lab please Calorimetry Post Lab Questions Specific heat of metal mass of water Study with quizlet and memorize flashcards containing terms like part a.1. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s). Calorimetry Post Lab Questions.
From www.chegg.com
Solved Calorimetry Post Lab Questions To receive full Calorimetry Post Lab Questions The study of heat energy, temperature, and heat transfer. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. This. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Calorimetry PostLab Report (a) Report the collected Calorimetry Post Lab Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Study with quizlet and memorize flashcards containing terms like part a.1. The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like carbohydrates,. This is the. Calorimetry Post Lab Questions.
From www.studocu.com
Post Lab 13 Calorimetry Heat of Reaction CHEM131L 3 010 Calorimetry Calorimetry Post Lab Questions Study with quizlet and memorize flashcards containing terms like part a.1. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Abstract the main. Calorimetry Post Lab Questions.
From www.chegg.com
CALORIMETRY Postlab Questions Date Name Partners) 1. Calorimetry Post Lab Questions Study with quizlet and memorize flashcards containing terms like part. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. Specific heat of metal mass of water This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Calorimetry Post Lab Questions To receive full Calorimetry Post Lab Questions The study of heat energy, temperature, and heat transfer. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Study with quizlet and memorize flashcards containing terms like carbohydrates,. This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Specific heat of metal mass of water In this. Calorimetry Post Lab Questions.
From www.studocu.com
Calorimetry Lab SE Lab General Physics 1 Studocu Calorimetry Post Lab Questions Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Specific heat of metal mass of water Study with quizlet and memorize flashcards containing terms like part. The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the. Calorimetry Post Lab Questions.
From www.chegg.com
Solved CALORIMETRY Postlab Questions Date Name Partners) 1. Calorimetry Post Lab Questions Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like part. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. Calorimetry Post Lab Questions.
From www.chegg.com
Section Name EXPERIMENT 8 CALORIMETRY Postlab Calorimetry Post Lab Questions Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. This is. Calorimetry Post Lab Questions.
From www.chegg.com
Solved PostLab Questions 1. What does the calorimeter Calorimetry Post Lab Questions Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. Study with quizlet and memorize flashcards containing terms like carbohydrates,. The study of heat energy, temperature, and heat transfer. Specific heat of metal mass of water In this set of practice questions, we will go. Calorimetry Post Lab Questions.
From www.chegg.com
CALORIMETRY Postlab Questions Partner(s) Date Name Calorimetry Post Lab Questions This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Study with quizlet and memorize flashcards containing terms like part. Study with quizlet and memorize flashcards containing terms like part a.1. Study with quizlet and memorize flashcards containing terms like carbohydrates,. Specific heat of metal mass of water Therefore, the enthalpy. Calorimetry Post Lab Questions.
From www.numerade.com
SOLVED Postlab Questions Heat is lost to the Styrofoam calorimeter Calorimetry Post Lab Questions In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Study with quizlet and memorize flashcards containing terms like part a.1. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Study with quizlet and memorize flashcards containing terms like. Calorimetry Post Lab Questions.
From www.chegg.com
CALORIMETRY Postlab Questions Date Name Partners) 1. Calorimetry Post Lab Questions Specific heat of metal mass of water Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Study with quizlet and memorize flashcards containing terms like carbohydrates,. The study of heat energy, temperature, and heat transfer. Study with quizlet and memorize flashcards containing terms like part. This is the post lab for experiment 25 which was the. Calorimetry Post Lab Questions.
From www.chegg.com
Solved Calorimetry Post Lab (10 pts) An experiment is to be Calorimetry Post Lab Questions Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Study with quizlet and memorize flashcards containing terms like carbohydrates,. The study of heat energy, temperature, and heat transfer. Therefore, the enthalpy of the solution, ∆hs, would be reported as too high. Question 1 the energy in reaction 1,. Calorimetry Post Lab Questions.
From www.chegg.com
Solved 59 CALORIMETRY CHM 1045LICHM 1046L POST LAB NAME Calorimetry Post Lab Questions Specific heat of metal mass of water Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Study. Calorimetry Post Lab Questions.
From www.chegg.com
Solved CHM 2045L Heat Effects and Calorimetry PostLab Calorimetry Post Lab Questions This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Question 1 the energy in reaction 1, x 1 , represents the energy released when about 1 g of naoh (s) is dissolved in water. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity,. Calorimetry Post Lab Questions.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Post Lab Questions Study with quizlet and memorize flashcards containing terms like carbohydrates,. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction, finding the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. Question 1. Calorimetry Post Lab Questions.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Calorimetry Post Lab Questions The study of heat energy, temperature, and heat transfer. Specific heat of metal mass of water Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a calorimeter. This is the post lab for experiment 25 which was the calorimetry experiment for general chemistry 1 with lab. Therefore, the enthalpy of. Calorimetry Post Lab Questions.