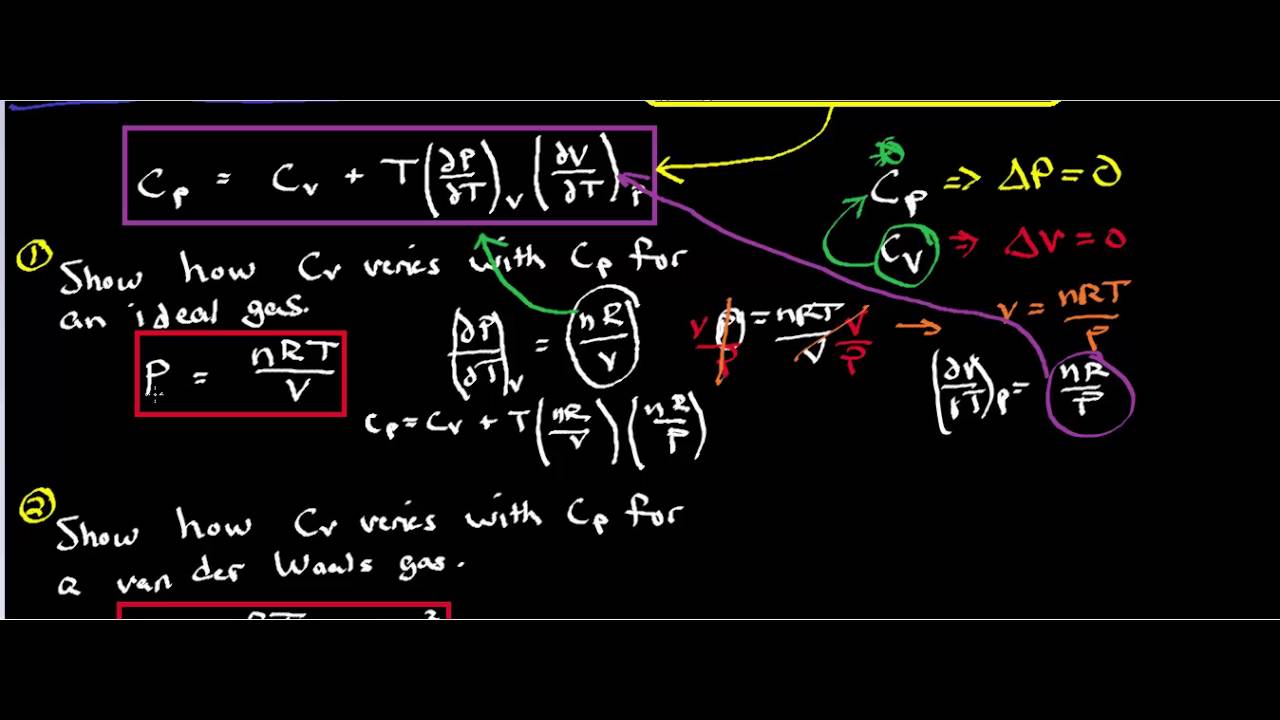
Cv Value In Thermodynamics . Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. This rather remarkable result has been. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Specific heat is the heat energy required to. Cv and cp are two terms used in thermodynamics. Cp = cv + r.
from www.youtube.com
Cp = cv + r. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. This rather remarkable result has been. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Specific heat is the heat energy required to. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. Cv and cp are two terms used in thermodynamics.
Relating Heat Capacities Cp and Cv YouTube
Cv Value In Thermodynamics Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Cp = cv + r. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. Cv and cp are two terms used in thermodynamics. This rather remarkable result has been. Specific heat is the heat energy required to. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant.
From theinstrumentguru.com
Control valve sizing calculator Cv Calculator Cv Value In Thermodynamics Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Cp = cv + r. Cv and cp are two terms used in thermodynamics. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. The specific heat constants for constant pressure and constant volume processes are related to the. Cv Value In Thermodynamics.
From alexandriadentaljournal.com
How To Calculate Cp And Cv In Thermodynamics Curriculum Vitae Template Cv Value In Thermodynamics Specific heat is the heat energy required to. This rather remarkable result has been. Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Cp = cv + r. For a monatomic ideal gas, cp = cv. Cv Value In Thermodynamics.
From www.youtube.com
👉🏻CpCv=V ,Thermodynamics Chapter 🤩🔥BSC 2nd Year💯 Physical Chemistry Cv Value In Thermodynamics The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. Cp = cv + r. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Cv and cp are two terms used in thermodynamics. For. Cv Value In Thermodynamics.
From www.researchgate.net
Isotherms, Thermodynamic and parameters values of CV Parameters Cv Value In Thermodynamics Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. Cv and cp are two terms used in thermodynamics. This rather remarkable result has been. Cp = cv + r. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or. Cv Value In Thermodynamics.
From slideplayer.com
First Law of Thermodynamics ppt download Cv Value In Thermodynamics Cv and cp are two terms used in thermodynamics. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. The specific heat constants. Cv Value In Thermodynamics.
From www.toppr.com
Cv, respectively, If gamma = CpCv and R is the universal gas constant Cv Value In Thermodynamics Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Cp = cv + r. This rather remarkable result has been. Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. Isobaric specific heat (cp ) is used for air. Cv Value In Thermodynamics.
From www1.grc.nasa.gov
Specific Heats cp and cv Glenn Research Center NASA Cv Value In Thermodynamics Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. Cp = cv + r. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats,. Cv Value In Thermodynamics.
From byjus.com
derive Cp Cv= Cv Value In Thermodynamics The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). 47 rows in thermal physics and thermodynamics, the. Cv Value In Thermodynamics.
From id.hutomosungkar.com
46+ How To Calculate The Cv Today Hutomo Cv Value In Thermodynamics Specific heat is the heat energy required to. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given. Cv Value In Thermodynamics.
From www.youtube.com
Specific Heat capacities of gas Cp and Cv First law of Cv Value In Thermodynamics This rather remarkable result has been. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. In thermodynamics, the heat capacity. Cv Value In Thermodynamics.
From www.youtube.com
Derive Cp Cv = R Thermodynamics BSc 3rd Semester Physics AP Cv Value In Thermodynamics 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. This rather remarkable result has been. The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. Specific heat is the heat energy required to. In. Cv Value In Thermodynamics.
From pediaa.com
Difference Between CV and CP Definition, Properties, Formula Cv Value In Thermodynamics For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. 47 rows in thermal physics and thermodynamics, the heat capacity ratio,. Cv Value In Thermodynamics.
From www.youtube.com
comparision between cv and ct in thermodynamics thermodynamics Cv Value In Thermodynamics Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Cp = cv + r. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). For a monatomic ideal gas, cp = cv + r = 3 2r + r =. Cv Value In Thermodynamics.
From byjus.com
The value of gamma=Cp/Cv is 4/3 for an adiabatic process of an ideal Cv Value In Thermodynamics 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Cp = cv + r. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. For a monatomic ideal gas, cp = cv. Cv Value In Thermodynamics.
From www.youtube.com
CP CV=R Class 11 Chemistry Thermodynamics YouTube Cv Value In Thermodynamics Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats,. Cv Value In Thermodynamics.
From byjus.com
cp/cv=R,how Cv Value In Thermodynamics In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. The specific heat constants for constant pressure and constant volume processes are related. Cv Value In Thermodynamics.
From www.youtube.com
Thermodynamics Specific heat Cp vs Cv relationship (Part 2) Class Cv Value In Thermodynamics Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Specific heat is the heat energy required to. Cp = cv + r. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. For a monatomic ideal gas, cp = cv + r = 3 2r + r =. Cv Value In Thermodynamics.
From www.grc.nasa.gov
Specific Heats Cv Value In Thermodynamics This rather remarkable result has been. Cv and cp are two terms used in thermodynamics. Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Specific heat (c) is. Cv Value In Thermodynamics.
From www.youtube.com
CpCv relations in thermodynamics Specific heats relations derivation Cv Value In Thermodynamics The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Cv and cp are two terms used in thermodynamics. Isobaric specific heat (cp ) is. Cv Value In Thermodynamics.
From classnotes.org.in
Heat Capacity Chemistry, Class 11, Thermodynamics Cv Value In Thermodynamics Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of. Cv Value In Thermodynamics.
From quizdialogizes.z4.web.core.windows.net
What Is Cp And Cv In Thermodynamics Cv Value In Thermodynamics 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Cv and cp are two terms used in thermodynamics. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Specific heat is the heat energy required to. Specific heat (c). Cv Value In Thermodynamics.
From www.youtube.com
Heat Capacity at Constant Volume (Cv) and at Pressure(Cp) Relation Cv Value In Thermodynamics Cv and cp are two terms used in thermodynamics. The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. Cp = cv + r. This rather remarkable result has been. Cv is the specific. Cv Value In Thermodynamics.
From physics.stackexchange.com
thermodynamics Derivation of heat capacity at constant pressure and Cv Value In Thermodynamics Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Specific heat is the heat energy required to. Cp = cv + r. Cv and cp are two terms used in thermodynamics. Cv is the specific heat at constant volume, and cp is the specific heat at. Cv Value In Thermodynamics.
From www.youtube.com
Prove Cp Cv = R ; where Cp & Cv are the molar specific heat of a gas Cv Value In Thermodynamics 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. Specific heat is the heat. Cv Value In Thermodynamics.
From www.youtube.com
Relationship between Cp and Cv Cp Cv = R Specific Heat at Cv Value In Thermodynamics Specific heat is the heat energy required to. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Cp = cv + r.. Cv Value In Thermodynamics.
From www.semanticscholar.org
Figure 7 from Molar Heat Capacity (Cv) for Saturated and Compressed Cv Value In Thermodynamics Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. Specific heat is the heat energy required to. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant.. Cv Value In Thermodynamics.
From www.slideserve.com
PPT Thermodynamics I Chapter 2 Properties of Pure Substances Cv Value In Thermodynamics Cv and cp are two terms used in thermodynamics. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. This rather remarkable result has been. In thermodynamics, the heat capacity at constant volume, , and the heat capacity. Cv Value In Thermodynamics.
From www.studypool.com
SOLUTION Heat and First Law of Thermodynamics Prove that Cp Cv = R Cv Value In Thermodynamics Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. Cp = cv + r. For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r. Cv Value In Thermodynamics.
From www.youtube.com
Why is Cp = Cv + R ?? // Thermodynamics Class 100 YouTube Cv Value In Thermodynamics Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. Cv and cp are two terms used in thermodynamics. The specific heat. Cv Value In Thermodynamics.
From quizdialogizes.z4.web.core.windows.net
What Is Cp And Cv In Thermodynamics Cv Value In Thermodynamics Cv and cp are two terms used in thermodynamics. Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. In thermodynamics, the heat capacity at constant volume, , and the heat capacity at constant. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). This rather remarkable. Cv Value In Thermodynamics.
From www.youtube.com
Thermodynamics Mayer's Formula Derivation CpCv=R Proof YouTube Cv Value In Thermodynamics The specific heat constants for constant pressure and constant volume processes are related to the gas constant for a given gas. Cp = cv + r. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). Specific heat (c) is the amount of heat required to change the temperature of a mass unit of. Cv Value In Thermodynamics.
From www.youtube.com
Relating Heat Capacities Cp and Cv YouTube Cv Value In Thermodynamics Cv and cp are two terms used in thermodynamics. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. Cv is the specific heat at constant volume, and cp is. Cv Value In Thermodynamics.
From www.youtube.com
Thermodynamics SPECIFIC HEATS cv & cp in 12 Minutes! YouTube Cv Value In Thermodynamics For a monatomic ideal gas, cp = cv + r = 3 2r + r = 5 2r (one mole of a monatomic ideal gas) the heat capacity functions have a pivotal. 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. The specific heat constants. Cv Value In Thermodynamics.
From www.slideserve.com
PPT THERMODYNAMIC PowerPoint Presentation, free download ID6381537 Cv Value In Thermodynamics Cv is the specific heat at constant volume, and cp is the specific heat at constant pressure. Specific heat (c) is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). For a monatomic ideal. Cv Value In Thermodynamics.
From www.youtube.com
Derivation of Cp Cv = R Mayer's Formula using First Law of Cv Value In Thermodynamics Specific heat is the heat energy required to. Isobaric specific heat (cp ) is used for air in a constant pressure (δp = 0). 47 rows in thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or laplace's. For a monatomic ideal gas, cp = cv + r = 3. Cv Value In Thermodynamics.