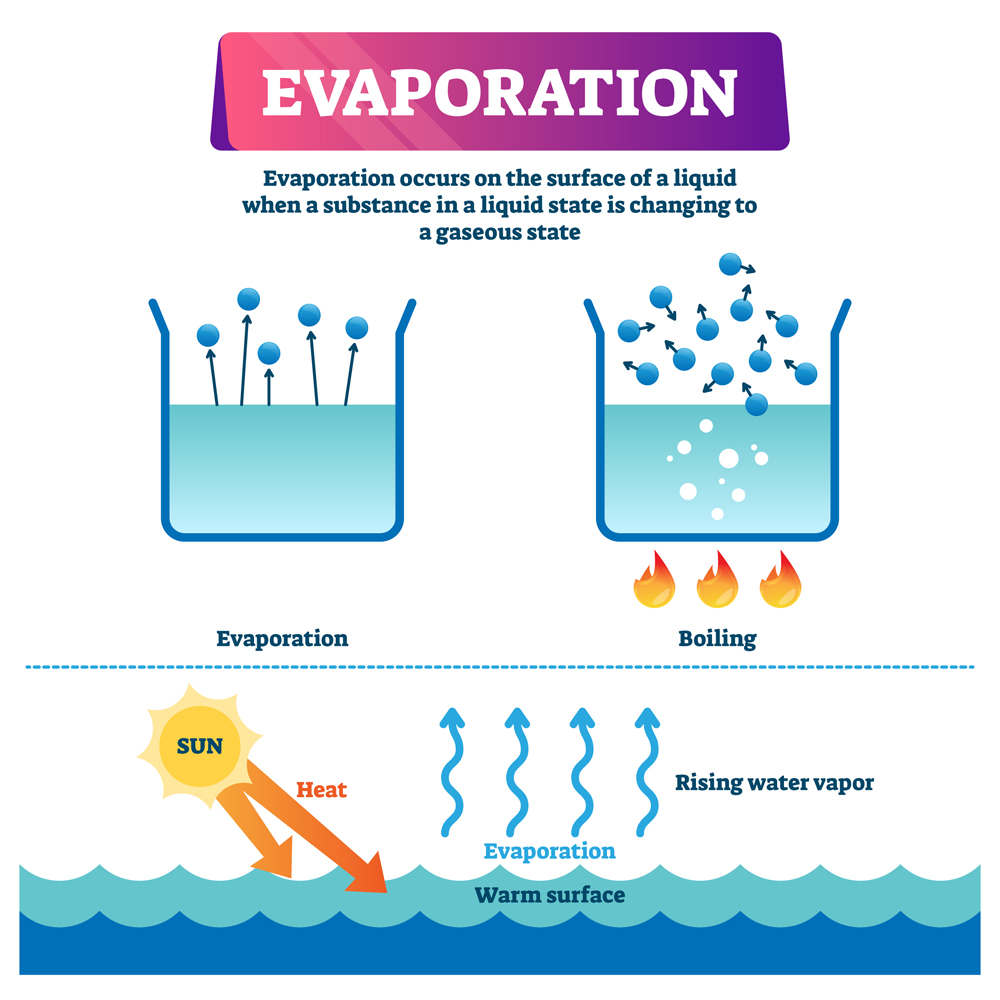
Why Partially Evaporate Rather Than Evaporation To Dryness . Evaporation is when a liquid is heated and changes state into a gas. We simply heat the solution until all the water boils off. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. (i) how would you know when excess strontium carbonate had been added in step 1? Evaporation to dryness is the more brainless method. This will leave behind the solid. The copper sulfate turns into dry crystals when all the water has evaporated. The copper sulfate (the solute) does not evaporate. The water (the solvent) heats up and evaporates. Evaporation, on the other hand, is a relatively fast and scalable. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. When a solution is heated, some of the solvent evaporates, leaving behind a saturated.
from www.scienceabc.com
The copper sulfate (the solute) does not evaporate. (i) how would you know when excess strontium carbonate had been added in step 1? When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Evaporation is when a liquid is heated and changes state into a gas. The copper sulfate turns into dry crystals when all the water has evaporated. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. We simply heat the solution until all the water boils off. Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. This will leave behind the solid.
Why Does Water Evaporate At Room Temperature?
Why Partially Evaporate Rather Than Evaporation To Dryness Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. (i) how would you know when excess strontium carbonate had been added in step 1? Evaporation, on the other hand, is a relatively fast and scalable. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The copper sulfate (the solute) does not evaporate. The copper sulfate turns into dry crystals when all the water has evaporated. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). We simply heat the solution until all the water boils off. Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. [1] (ii) why is it. Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. The water (the solvent) heats up and evaporates. When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Evaporation to dryness is the more brainless method. This will leave behind the solid. Evaporation is when a liquid is heated and changes state into a gas.
From www.scienceabc.com
Are Evaporation And Boiling The Same? » Science ABC Why Partially Evaporate Rather Than Evaporation To Dryness Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. This will leave behind the solid. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size,. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
Separation of Mixtures ppt download Why Partially Evaporate Rather Than Evaporation To Dryness Evaporation to dryness is the more brainless method. Evaporation, on the other hand, is a relatively fast and scalable. (i) how would you know when excess strontium carbonate had been added in step 1? We simply heat the solution until all the water boils off. Crystallization is preferred over evaporation to dryness when you want to get pure and specific. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.worldatlas.com
The Water Cycle WorldAtlas Why Partially Evaporate Rather Than Evaporation To Dryness [1] (ii) why is it. We simply heat the solution until all the water boils off. Evaporation to dryness is the more brainless method. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. The water (the solvent) heats up and evaporates. Evaporation. Why Partially Evaporate Rather Than Evaporation To Dryness.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk Why Partially Evaporate Rather Than Evaporation To Dryness The water (the solvent) heats up and evaporates. This will leave behind the solid. We simply heat the solution until all the water boils off. Evaporation is when a liquid is heated and changes state into a gas. Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. Evaporation to dryness is the more brainless. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.youtube.com
Separation of solid liquid mixturesEvaporation to dryness YouTube Why Partially Evaporate Rather Than Evaporation To Dryness [1] (ii) why is it. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. Evaporation, on the other hand, is a relatively fast and scalable. (i) how would you know when excess strontium carbonate had been added in step 1? This will. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
Evaporation works like this ppt download Why Partially Evaporate Rather Than Evaporation To Dryness 5 dry the crystals between filter papers. (i) how would you know when excess strontium carbonate had been added in step 1? Evaporation is when a liquid is heated and changes state into a gas. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). Evaporation requires heat (or air movement above the sample) to. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
Separating Mixtures. ppt download Why Partially Evaporate Rather Than Evaporation To Dryness The copper sulfate turns into dry crystals when all the water has evaporated. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). When a solution is heated,. Why Partially Evaporate Rather Than Evaporation To Dryness.
From loegjniwa.blob.core.windows.net
Does Kerosene Evaporate Faster Than Water at Janine Mohan blog Why Partially Evaporate Rather Than Evaporation To Dryness Evaporation is when a liquid is heated and changes state into a gas. (i) how would you know when excess strontium carbonate had been added in step 1? This will leave behind the solid. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.teachoo.com
Why are we able to sip hot tea or milk faster from a saucer rather Why Partially Evaporate Rather Than Evaporation To Dryness [1] (ii) why is it. The copper sulfate turns into dry crystals when all the water has evaporated. We simply heat the solution until all the water boils off. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. Evaporation, on the other. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
THE HYDROSPHERE. ppt download Why Partially Evaporate Rather Than Evaporation To Dryness The copper sulfate (the solute) does not evaporate. Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. The water (the solvent) heats up and evaporates. The copper sulfate turns into dry crystals when all the water has evaporated. Liquid water is made up of molecules of h 2 o attracted to one another by. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.teachoo.com
11+ Examples of Evaporation in our daily life (Explained!) Teachoo Why Partially Evaporate Rather Than Evaporation To Dryness The copper sulfate turns into dry crystals when all the water has evaporated. Evaporation is when a liquid is heated and changes state into a gas. We simply heat the solution until all the water boils off. The water (the solvent) heats up and evaporates. If the substance is a solid mixed in a solvent, begin by filtering (or decanting).. Why Partially Evaporate Rather Than Evaporation To Dryness.
From quizlet.com
Evaporation; separating a soluble solid and a solution Diagram Quizlet Why Partially Evaporate Rather Than Evaporation To Dryness Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. (i) how would you know when excess strontium carbonate had been added in step 1? When a solution is heated, some of the solvent evaporates, leaving behind a saturated. We simply heat the. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Why Partially Evaporate Rather Than Evaporation To Dryness [1] (ii) why is it. Evaporation, on the other hand, is a relatively fast and scalable. (i) how would you know when excess strontium carbonate had been added in step 1? The copper sulfate (the solute) does not evaporate. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.slideserve.com
PPT Drying PowerPoint Presentation, free download ID1355007 Why Partially Evaporate Rather Than Evaporation To Dryness (i) how would you know when excess strontium carbonate had been added in step 1? Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). When a solution is heated, some of the. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Why Partially Evaporate Rather Than Evaporation To Dryness 5 dry the crystals between filter papers. When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Evaporation is when a liquid is heated and changes state into a gas. The copper sulfate turns into dry crystals when all the water has evaporated. This will leave behind the solid. (i) how would you know when excess. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.slideserve.com
PPT Water and Changes of State PowerPoint Presentation, free download Why Partially Evaporate Rather Than Evaporation To Dryness 5 dry the crystals between filter papers. Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. [1] (ii) why is it. Evaporation, on the other hand, is. Why Partially Evaporate Rather Than Evaporation To Dryness.
From chemnotcheem.com
Differences between crystallisation and evaporation to dryness Why Partially Evaporate Rather Than Evaporation To Dryness 5 dry the crystals between filter papers. The copper sulfate (the solute) does not evaporate. When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. Evaporation to dryness is the more brainless method. Evaporation, on the other hand,. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
EVAPORATION Definition Process by which water is changed from the Why Partially Evaporate Rather Than Evaporation To Dryness 5 dry the crystals between filter papers. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). This will leave behind the solid. The water (the solvent) heats up and evaporates. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size,. Why Partially Evaporate Rather Than Evaporation To Dryness.
From loercbzqq.blob.core.windows.net
Explain Evaporation Energy at Marvin Dudley blog Why Partially Evaporate Rather Than Evaporation To Dryness The copper sulfate (the solute) does not evaporate. This will leave behind the solid. [1] (ii) why is it. We simply heat the solution until all the water boils off. Evaporation, on the other hand, is a relatively fast and scalable. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). Evaporation to dryness is. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.vedantu.com
Evaporation Learn Important Terms and Concepts Why Partially Evaporate Rather Than Evaporation To Dryness Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. The water (the solvent) heats up and evaporates. 5 dry the crystals between filter papers. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation, on the other hand,. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.youtube.com
Evaporation to dryness & crystallization oleval YouTube Why Partially Evaporate Rather Than Evaporation To Dryness The copper sulfate (the solute) does not evaporate. This will leave behind the solid. Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. Evaporation to dryness is the more brainless method. When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Evaporation, on the other hand, is. Why Partially Evaporate Rather Than Evaporation To Dryness.
From exyukbtsg.blob.core.windows.net
Is Boiling Water Evaporation at Lawrence Edwards blog Why Partially Evaporate Rather Than Evaporation To Dryness Evaporation to dryness is the more brainless method. (i) how would you know when excess strontium carbonate had been added in step 1? When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. 5. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
Separation Techniques ppt download Why Partially Evaporate Rather Than Evaporation To Dryness The water (the solvent) heats up and evaporates. Evaporation, on the other hand, is a relatively fast and scalable. Evaporation to dryness is the more brainless method. Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. The copper sulfate (the solute) does not evaporate. [1] (ii) why is it. Crystallization offers control over crystal. Why Partially Evaporate Rather Than Evaporation To Dryness.
From temperaturemaster.com
Exploring the Evaporation Process of Dry Ice Why Partially Evaporate Rather Than Evaporation To Dryness This will leave behind the solid. Evaporation to dryness is the more brainless method. Evaporation requires heat (or air movement above the sample) to drive off a volatile solvent. [1] (ii) why is it. We simply heat the solution until all the water boils off. (i) how would you know when excess strontium carbonate had been added in step 1?. Why Partially Evaporate Rather Than Evaporation To Dryness.
From serc.carleton.edu
Evapotranspiration and Crop Water Use Why Partially Evaporate Rather Than Evaporation To Dryness Evaporation to dryness is the more brainless method. The copper sulfate turns into dry crystals when all the water has evaporated. When a solution is heated, some of the solvent evaporates, leaving behind a saturated. If the substance is a solid mixed in a solvent, begin by filtering (or decanting). The water (the solvent) heats up and evaporates. 5 dry. Why Partially Evaporate Rather Than Evaporation To Dryness.
From celgbrvg.blob.core.windows.net
How Does Water Evaporate At Night at Rita Guerra blog Why Partially Evaporate Rather Than Evaporation To Dryness [1] (ii) why is it. The copper sulfate turns into dry crystals when all the water has evaporated. Evaporation to dryness is the more brainless method. The copper sulfate (the solute) does not evaporate. We simply heat the solution until all the water boils off. Evaporation, on the other hand, is a relatively fast and scalable. If the substance is. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.teachoo.com
Evaporation Meaning and Factors affecting it Teachoo Why Partially Evaporate Rather Than Evaporation To Dryness The copper sulfate turns into dry crystals when all the water has evaporated. The copper sulfate (the solute) does not evaporate. We simply heat the solution until all the water boils off. Evaporation to dryness is the more brainless method. This will leave behind the solid. Crystallization is preferred over evaporation to dryness when you want to get pure and. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
Science Year 3/4A Summer 1 ppt download Why Partially Evaporate Rather Than Evaporation To Dryness The water (the solvent) heats up and evaporates. 5 dry the crystals between filter papers. Evaporation, on the other hand, is a relatively fast and scalable. We simply heat the solution until all the water boils off. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their. Why Partially Evaporate Rather Than Evaporation To Dryness.
From quizunderslung.z4.web.core.windows.net
What Is Evaporation To Dryness Why Partially Evaporate Rather Than Evaporation To Dryness Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. [1] (ii) why is it. 5 dry the crystals between filter papers. The water (the solvent) heats up and evaporates. This will leave behind the solid. The copper sulfate turns into dry crystals when all the water has evaporated. We simply heat. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.slideserve.com
PPT Separation Techniques PowerPoint Presentation, free download ID Why Partially Evaporate Rather Than Evaporation To Dryness Evaporation to dryness is the more brainless method. Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. This will leave behind the solid. The. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.teachoo.com
Why is Evaporation called a surface phenomenon? Teachoo Why Partially Evaporate Rather Than Evaporation To Dryness We simply heat the solution until all the water boils off. The water (the solvent) heats up and evaporates. When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. 5 dry the crystals between filter papers. Evaporation, on. Why Partially Evaporate Rather Than Evaporation To Dryness.
From www.chemicals.co.uk
What is the Definition of Evaporation in Chemistry? Why Partially Evaporate Rather Than Evaporation To Dryness 5 dry the crystals between filter papers. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. The water (the solvent). Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
States of Matter Chapter ppt download Why Partially Evaporate Rather Than Evaporation To Dryness Crystallization offers control over crystal size and morphology, making it suitable for industries that require specific crystal properties. [1] (ii) why is it. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Evaporation to dryness is the more brainless method. The copper sulfate turns into dry crystals. Why Partially Evaporate Rather Than Evaporation To Dryness.
From slideplayer.com
The Water Cycle. ppt download Why Partially Evaporate Rather Than Evaporation To Dryness When a solution is heated, some of the solvent evaporates, leaving behind a saturated. Crystallization is preferred over evaporation to dryness when you want to get pure and specific crystals, as it provides better control over their size, shape, and purity. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known. Why Partially Evaporate Rather Than Evaporation To Dryness.
From collegedunia.com
Factor Affecting Evaporation Definition, Rate of Evaporation, Examples Why Partially Evaporate Rather Than Evaporation To Dryness If the substance is a solid mixed in a solvent, begin by filtering (or decanting). The copper sulfate turns into dry crystals when all the water has evaporated. (i) how would you know when excess strontium carbonate had been added in step 1? Evaporation, on the other hand, is a relatively fast and scalable. This will leave behind the solid.. Why Partially Evaporate Rather Than Evaporation To Dryness.