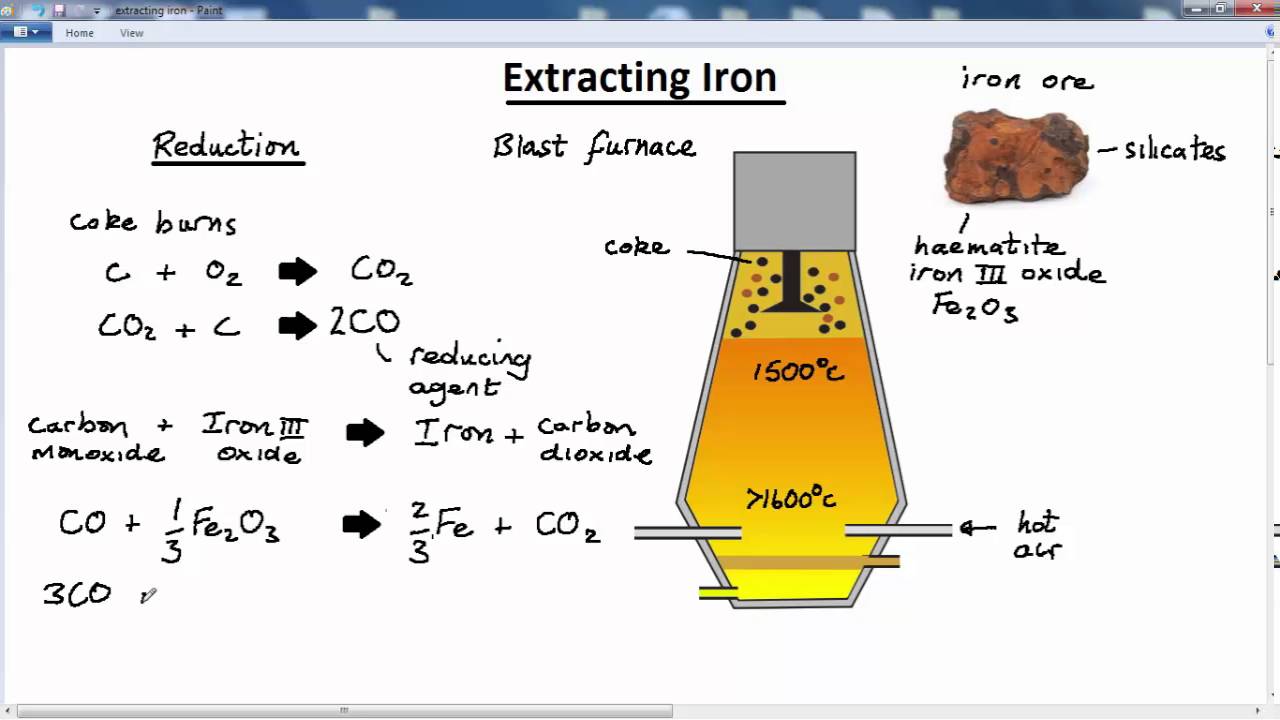
Iron + Steam Chemical Equation . why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Hydrogen passed over hot triiron tetroxide reduces it to iron. Write the chemical formula for steam: The reaction occurs as follows: iron reacts with steam to form iron oxide and hydrogen gas. Fe + h2o → fe3o4 + h2 this equation shows. Write a balanced equation for the. derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to form iron (ii,iii) oxide and hydrogen gas. the hydrogen produced in the reaction is swept away by the stream of steam. Under different conditions, the products of this reaction will also react together. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. Write the chemical formula for iron: now, we can write the balanced equation for the reaction between iron and steam: 3 fe (s) + 4 h 2 o (g) → fe 3 o 4.
from www.youtube.com
why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? the hydrogen produced in the reaction is swept away by the stream of steam. now, we can write the balanced equation for the reaction between iron and steam: Fe + h2o → fe3o4 + h2 this equation shows. Write the chemical formula for iron: Write the chemical formula for steam: Hydrogen passed over hot triiron tetroxide reduces it to iron. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. The reaction occurs as follows: iron reacts with steam to form iron oxide and hydrogen gas.
GCSE CHEMISTRY REACTIVITY SERIES LESSON 10 exraction iron YouTube
Iron + Steam Chemical Equation Under different conditions, the products of this reaction will also react together. now, we can write the balanced equation for the reaction between iron and steam: derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to form iron (ii,iii) oxide and hydrogen gas. the hydrogen produced in the reaction is swept away by the stream of steam. Under different conditions, the products of this reaction will also react together. 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. Write the chemical formula for iron: why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Write a balanced equation for the. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. The reaction occurs as follows: iron reacts with steam to form iron oxide and hydrogen gas. Fe + h2o → fe3o4 + h2 this equation shows. Write the chemical formula for steam: Hydrogen passed over hot triiron tetroxide reduces it to iron.
From byjus.com
what is the formula of rusting of iron Iron + Steam Chemical Equation Write the chemical formula for steam: now, we can write the balanced equation for the reaction between iron and steam: Write the chemical formula for iron: derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to form iron (ii,iii) oxide and hydrogen gas. The reaction occurs as follows: Fe + h2o → fe3o4. Iron + Steam Chemical Equation.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron + Steam Chemical Equation the hydrogen produced in the reaction is swept away by the stream of steam. Hydrogen passed over hot triiron tetroxide reduces it to iron. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. Write the chemical formula for. Iron + Steam Chemical Equation.
From www.teachoo.com
Write equations for reaction of (i) iron with steam Class 10 Science Iron + Steam Chemical Equation Write the chemical formula for iron: Write a balanced equation for the. The reaction occurs as follows: Under different conditions, the products of this reaction will also react together. derive chemical equations from narrative descriptions of chemical reactions. 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. Hydrogen passed over hot triiron tetroxide reduces. Iron + Steam Chemical Equation.
From www.numerade.com
SOLVED Write balanced chemical equations for the following reactions. (a) The reaction of Iron + Steam Chemical Equation 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Under different conditions, the products of this reaction will also react together. iron reacts with steam to form iron. Iron + Steam Chemical Equation.
From www.youtube.com
GCSE CHEMISTRY REACTIVITY SERIES LESSON 10 exraction iron YouTube Iron + Steam Chemical Equation the hydrogen produced in the reaction is swept away by the stream of steam. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. Fe. Iron + Steam Chemical Equation.
From onlinesciencenotes.com
Chemical reactions, balanced and unbalanced chemical equations Online Science Notes Iron + Steam Chemical Equation The reaction occurs as follows: derive chemical equations from narrative descriptions of chemical reactions. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. the hydrogen produced in the reaction is swept away by the stream of steam.. Iron + Steam Chemical Equation.
From brainly.in
Iron reacts with steam to produce triferric tetraoxide and hydrogen write equation and balanced Iron + Steam Chemical Equation iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. Hydrogen passed over hot triiron tetroxide reduces it to iron. Write a balanced equation for the. The reaction occurs as follows: Write the chemical formula for iron: now, we. Iron + Steam Chemical Equation.
From www.slideshare.net
Chemical Reactions Iron + Steam Chemical Equation iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. the hydrogen produced in the reaction is swept away by the stream of steam. now, we can write the balanced equation for the reaction between iron and steam:. Iron + Steam Chemical Equation.
From www.numerade.com
SOLVEDwrite the chemical equation for the reaction was description is given below (a) iron Iron + Steam Chemical Equation why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to form iron oxide and hydrogen gas. Write the chemical formula for iron: iron has three different oxides,. Iron + Steam Chemical Equation.
From askfilo.com
5. Write balanced equations for the reaction of a. Iron with steam. Name.. Iron + Steam Chemical Equation iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. iron reacts with steam to form iron oxide and hydrogen gas. Hydrogen passed over hot triiron tetroxide reduces it to iron. derive chemical equations from narrative descriptions of. Iron + Steam Chemical Equation.
From www.slideserve.com
PPT Introductory Chemistry , 3 rd Edition Nivaldo Tro PowerPoint Presentation ID243462 Iron + Steam Chemical Equation The reaction occurs as follows: the hydrogen produced in the reaction is swept away by the stream of steam. Write the chemical formula for steam: now, we can write the balanced equation for the reaction between iron and steam: derive chemical equations from narrative descriptions of chemical reactions. why is the product of the reaction between. Iron + Steam Chemical Equation.
From mammothmemory.net
Iron introduced to heat and steam turns to black iron oxide Iron + Steam Chemical Equation Write the chemical formula for iron: why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Under different conditions, the products of this reaction will also react together. Write the chemical formula for steam: Hydrogen passed over hot triiron tetroxide reduces it to iron.. Iron + Steam Chemical Equation.
From www.youtube.com
Write equations for the reactions of (i) iron with steam (ii) calcium and potassium with water Iron + Steam Chemical Equation the hydrogen produced in the reaction is swept away by the stream of steam. Write the chemical formula for iron: now, we can write the balanced equation for the reaction between iron and steam: iron reacts with steam to form iron oxide and hydrogen gas. 3 fe (s) + 4 h 2 o (g) → fe 3. Iron + Steam Chemical Equation.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations Iron + Steam Chemical Equation Hydrogen passed over hot triiron tetroxide reduces it to iron. Write the chemical formula for iron: Fe + h2o → fe3o4 + h2 this equation shows. derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to form iron (ii,iii) oxide and hydrogen gas. iron reacts with steam to form iron oxide and hydrogen. Iron + Steam Chemical Equation.
From www.numerade.com
SOLVED (a) Write the balanced chemical equations for the following reactions (i) Action of Iron + Steam Chemical Equation Fe + h2o → fe3o4 + h2 this equation shows. Write the chemical formula for iron: why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? The reaction occurs as follows: now, we can write the balanced equation for the reaction between iron. Iron + Steam Chemical Equation.
From www.numerade.com
SOLVED Write chemical equations for the reactions that occur when (a) Iron reacts with steam Iron + Steam Chemical Equation Write the chemical formula for iron: derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to form iron oxide and hydrogen gas. Hydrogen passed over hot triiron tetroxide reduces it to iron. The reaction occurs as follows: 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. why is. Iron + Steam Chemical Equation.
From www.toppr.com
OP Write equations the reactions of (i) iron with steam (ii) calcium and potassium with water 1 Iron + Steam Chemical Equation Write a balanced equation for the. now, we can write the balanced equation for the reaction between iron and steam: why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? 3 fe (s) + 4 h 2 o (g) → fe 3 o. Iron + Steam Chemical Equation.
From www.toppr.com
Explain with the help of chemical equations what happens when (a) Copper is heated strongly Iron + Steam Chemical Equation 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. the hydrogen produced in the reaction is swept away by the stream of steam. Write a balanced equation for the. Fe + h2o → fe3o4 + h2 this equation shows. Hydrogen passed over hot triiron tetroxide reduces it to iron. derive chemical equations from. Iron + Steam Chemical Equation.
From brainly.in
Write chemical equation reactions taking place when carried out with the help of (a) iron reacts Iron + Steam Chemical Equation Fe + h2o → fe3o4 + h2 this equation shows. Hydrogen passed over hot triiron tetroxide reduces it to iron. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. iron reacts with steam to form iron (ii,iii) oxide. Iron + Steam Chemical Equation.
From www.doubtnut.com
Write equations for the reactions. (i) Iron with steam (ii) Calciu Iron + Steam Chemical Equation now, we can write the balanced equation for the reaction between iron and steam: why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? derive chemical equations from narrative descriptions of chemical reactions. Fe + h2o → fe3o4 + h2 this equation. Iron + Steam Chemical Equation.
From www.youtube.com
Trick to remember Reactions of Rusting of iron/Electrochemistry/ASN CHEMISTRY YouTube Iron + Steam Chemical Equation the hydrogen produced in the reaction is swept away by the stream of steam. now, we can write the balanced equation for the reaction between iron and steam: 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to. Iron + Steam Chemical Equation.
From brainly.in
Write equations for the reactions of (i) iron with steam (ii) calcium and potassium with water Iron + Steam Chemical Equation The reaction occurs as follows: now, we can write the balanced equation for the reaction between iron and steam: Write a balanced equation for the. Under different conditions, the products of this reaction will also react together. Write the chemical formula for steam: iron reacts with steam to form iron (ii,iii) oxide and hydrogen gas. the hydrogen. Iron + Steam Chemical Equation.
From brainly.in
Write chemical equation reactions taking place when carried out with the help of (a) iron reacts Iron + Steam Chemical Equation Fe + h2o → fe3o4 + h2 this equation shows. iron reacts with steam to form iron (ii,iii) oxide and hydrogen gas. now, we can write the balanced equation for the reaction between iron and steam: Hydrogen passed over hot triiron tetroxide reduces it to iron. why is the product of the reaction between iron and steam. Iron + Steam Chemical Equation.
From learningschoolsonaj9w.z13.web.core.windows.net
Identifying Parts Of A Chemical Equation Iron + Steam Chemical Equation Fe + h2o → fe3o4 + h2 this equation shows. derive chemical equations from narrative descriptions of chemical reactions. now, we can write the balanced equation for the reaction between iron and steam: 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. The reaction occurs as follows: why is the product of. Iron + Steam Chemical Equation.
From www.youtube.com
Reactions of Iron Reactions Chemistry FuseSchool YouTube Iron + Steam Chemical Equation why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? Fe + h2o → fe3o4 + h2 this equation shows. Write the chemical formula for steam: Hydrogen passed over hot triiron tetroxide reduces it to iron. iron has three different oxides, and the. Iron + Steam Chemical Equation.
From www.slideserve.com
PPT Blast Furnace Reactions PowerPoint Presentation, free download ID469076 Iron + Steam Chemical Equation now, we can write the balanced equation for the reaction between iron and steam: Under different conditions, the products of this reaction will also react together. the hydrogen produced in the reaction is swept away by the stream of steam. Write the chemical formula for iron: Hydrogen passed over hot triiron tetroxide reduces it to iron. 3 fe. Iron + Steam Chemical Equation.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free download ID3552860 Iron + Steam Chemical Equation Write the chemical formula for iron: iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. The reaction occurs as follows: Hydrogen passed over hot triiron. Iron + Steam Chemical Equation.
From www.numerade.com
SOLVEDWrite balanced equations for (a) the reaction of iron with steam, (b) the reaction of Iron + Steam Chemical Equation Hydrogen passed over hot triiron tetroxide reduces it to iron. 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. The reaction occurs as follows: the hydrogen produced in the reaction is swept away by the stream of steam. Under different conditions, the products of this reaction will also react together. Write the chemical formula. Iron + Steam Chemical Equation.
From dxocqgdzl.blob.core.windows.net
Steam And Iron Reaction at Brenda McCarthy blog Iron + Steam Chemical Equation derive chemical equations from narrative descriptions of chemical reactions. Write the chemical formula for iron: Fe + h2o → fe3o4 + h2 this equation shows. The reaction occurs as follows: why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? the hydrogen. Iron + Steam Chemical Equation.
From www.youtube.com
Write equations for the reactions of(i) iron with steam (ii) calcium and potassium with water Iron + Steam Chemical Equation why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? the hydrogen produced in the reaction is swept away by the stream of steam. Write a balanced equation for the. iron has three different oxides, and the one formed when you pass. Iron + Steam Chemical Equation.
From www.slideserve.com
PPT Balancing Chemical Equations PowerPoint Presentation, free download ID6626399 Iron + Steam Chemical Equation derive chemical equations from narrative descriptions of chemical reactions. iron reacts with steam to form iron oxide and hydrogen gas. Write a balanced equation for the. Fe + h2o → fe3o4 + h2 this equation shows. now, we can write the balanced equation for the reaction between iron and steam: Hydrogen passed over hot triiron tetroxide reduces. Iron + Steam Chemical Equation.
From brainly.in
Write balanced chemical equations for the reactions of (i) iron with steam (ii) sodium and Iron + Steam Chemical Equation iron reacts with steam to form iron oxide and hydrogen gas. why is the product of the reaction between iron and steam is iron (ii, iii) oxide and not iron (ii) oxide or iron (iii) oxide? The reaction occurs as follows: Fe + h2o → fe3o4 + h2 this equation shows. the hydrogen produced in the reaction. Iron + Steam Chemical Equation.
From signalticket9.pythonanywhere.com
Spectacular Iron Oxide State Symbol Www Physics Wallah Com Notes Pdf Class 11 Iron + Steam Chemical Equation iron reacts with steam to form iron oxide and hydrogen gas. Write a balanced equation for the. derive chemical equations from narrative descriptions of chemical reactions. Under different conditions, the products of this reaction will also react together. the hydrogen produced in the reaction is swept away by the stream of steam. Hydrogen passed over hot triiron. Iron + Steam Chemical Equation.
From www.coursehero.com
[Solved] balanced chemical equation based on the following description iron... Course Hero Iron + Steam Chemical Equation Write a balanced equation for the. Fe + h2o → fe3o4 + h2 this equation shows. iron reacts with steam to form iron (ii,iii) oxide and hydrogen gas. 3 fe (s) + 4 h 2 o (g) → fe 3 o 4. Under different conditions, the products of this reaction will also react together. Write the chemical formula for. Iron + Steam Chemical Equation.
From chemistry.stackexchange.com
chemistry Reaction of iron with steam Chemistry Stack Exchange Iron + Steam Chemical Equation derive chemical equations from narrative descriptions of chemical reactions. iron has three different oxides, and the one formed when you pass steam over it, or when it is burned in air, is a black oxide, fe 3 o. Fe + h2o → fe3o4 + h2 this equation shows. now, we can write the balanced equation for the. Iron + Steam Chemical Equation.