Antacid Reaction Chemical Equation . In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. Other antacids, such as alka seltzer, work in similar ways. Titration of an antacid objective: The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment, you will standardize a solution of base using the analytical technique known as titration. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Below, show the mechanism and products for reaction in which.
from www.numerade.com
In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Other antacids, such as alka seltzer, work in similar ways. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Below, show the mechanism and products for reaction in which. Titration of an antacid objective: In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. In this experiment, you will standardize a solution of base using the analytical technique known as titration.
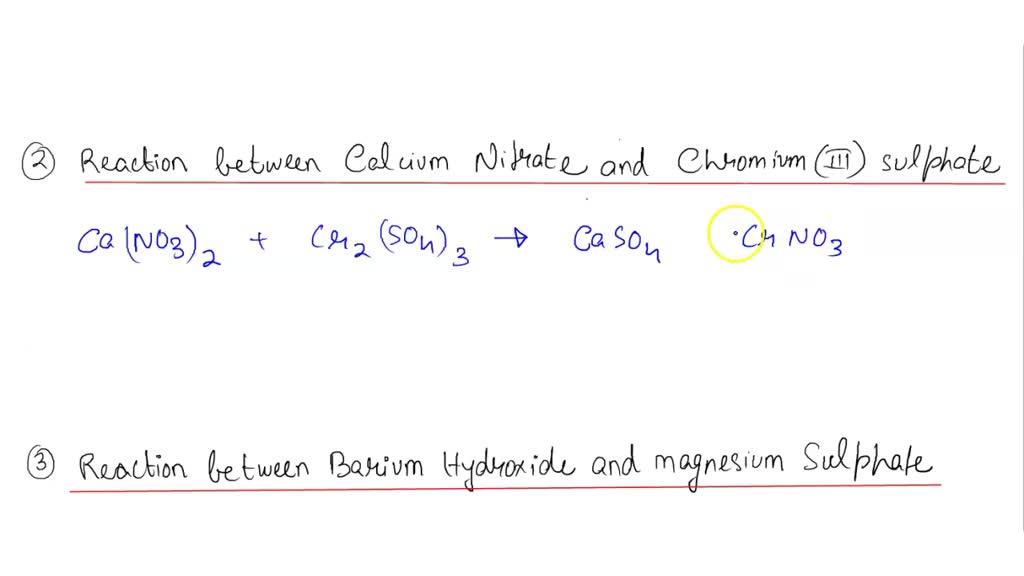
SOLVED 2. You can use a net ionic equation to predict whether a
Antacid Reaction Chemical Equation In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Titration of an antacid objective: The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. Other antacids, such as alka seltzer, work in similar ways. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Below, show the mechanism and products for reaction in which.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacid Reaction Chemical Equation Below, show the mechanism and products for reaction in which. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment, several. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED Write a balanced chemical equation for the neutralization Antacid Reaction Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the. Antacid Reaction Chemical Equation.
From brainly.com
write the neutralization equations that take place in the stomach with Antacid Reaction Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Titration of an antacid objective: The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment, you will standardize a solution of. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Reaction Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment, you will standardize a solution of base using the analytical technique known as titration. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID3824036 Antacid Reaction Chemical Equation In this experiment, you will standardize a solution of base using the analytical technique known as titration. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED Several brands of antacids use Al(OH)3 to react with stomach Antacid Reaction Chemical Equation In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. The balanced chemical equation for the reaction of the main ingredient of the. Antacid Reaction Chemical Equation.
From www.numerade.com
Antacids are compounds that neutralize stomach ac… Antacid Reaction Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Below, show the mechanism and products for reaction in which. Titration of an antacid objective: In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. A simple antacid tablet, milk. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVEDWrite the balanced chemical equation for the neutralization Antacid Reaction Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Other antacids, such as alka seltzer, work in similar ways. Below, show the mechanism and products for reaction in which. In this experiment we will analyze a typical antacid containing caco3, a base which will react with. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation, free download ID1994845 Antacid Reaction Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Titration of an antacid objective: A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Other antacids, such as alka seltzer, work in similar. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacid Reaction Chemical Equation Other antacids, such as alka seltzer, work in similar ways. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In today’s lab you. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT Antacids PowerPoint Presentation ID1994845 Antacid Reaction Chemical Equation Titration of an antacid objective: In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Below, show the mechanism and products for reaction in which. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT Drugs used in the treatment of peptic ulcer PowerPoint Antacid Reaction Chemical Equation Titration of an antacid objective: A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Below, show the mechanism and products for reaction in which. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and. Antacid Reaction Chemical Equation.
From slideplayer.com
Chapter 2 Antacids. ppt download Antacid Reaction Chemical Equation Below, show the mechanism and products for reaction in which. Titration of an antacid objective: In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. A simple antacid tablet, milk. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT Chemistry of antacids PowerPoint Presentation, free download ID Antacid Reaction Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In this experiment, you will standardize a solution of base using the analytical technique known as titration. Titration of an antacid objective: In today’s lab you will analyze antacids which are common medications used to neutralize stomach. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED Antacids are used to treat acid reflux by neutralizing stomach Antacid Reaction Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Other antacids, such as alka seltzer, work in similar ways. In this experiment, you will standardize a solution of base. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Reaction Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. Titration of an antacid objective: In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. Below, show the mechanism and products for reaction in. Antacid Reaction Chemical Equation.
From brainly.com
AlkaSeltzer® is an antacid. One of its active ingredients is NAHCO3 Antacid Reaction Chemical Equation In this experiment, you will standardize a solution of base using the analytical technique known as titration. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Below, show the mechanism and products for reaction in which. The balanced chemical equation for the reaction of the. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED Part A Write the balanced chemical equation for the Antacid Reaction Chemical Equation A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Below, show the mechanism and products for reaction in which. In this experiment, you will standardize a solution of base using the analytical technique known as titration. In this experiment we will analyze a typical antacid containing. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED Aluminum hydroxide in antacid tablets neutralize hydrochloric Antacid Reaction Chemical Equation The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to. Antacid Reaction Chemical Equation.
From www.slideshare.net
Antacids Antacid Reaction Chemical Equation Other antacids, such as alka seltzer, work in similar ways. Titration of an antacid objective: A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize. Antacid Reaction Chemical Equation.
From study.com
Writing Balanced Chemical Reactions Video & Lesson Transcript Antacid Reaction Chemical Equation Titration of an antacid objective: In this experiment, you will standardize a solution of base using the analytical technique known as titration. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In this experiment, several brands of antacids will be analyzed to determine the number of. Antacid Reaction Chemical Equation.
From slideplayer.com
Chapter 2 Antacids. ppt download Antacid Reaction Chemical Equation In this experiment, you will standardize a solution of base using the analytical technique known as titration. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment, several. Antacid Reaction Chemical Equation.
From www.slideserve.com
PPT If we ’ re going to do chemical reactions with antacids, we ’ d Antacid Reaction Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Other antacids, such as alka seltzer, work in similar ways. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment,. Antacid Reaction Chemical Equation.
From www.chegg.com
Solved One antacid tablet typically contains 600.mg of Antacid Reaction Chemical Equation In this experiment, you will standardize a solution of base using the analytical technique known as titration. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED 1. Calcium carbonate (CaCO3), a calcium supplement and the Antacid Reaction Chemical Equation In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. Below, show the mechanism and products for reaction in which. In this experiment we will analyze a typical antacid containing caco3,. Antacid Reaction Chemical Equation.
From www.numerade.com
Antacid Fizz When an antacid tablet dissolves in water, the fizz is due Antacid Reaction Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. Below, show the mechanism and products for reaction in which. A simple antacid tablet, milk of magnesia, has the following. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED 1. Write the chemical equation for the reaction of an antacid Antacid Reaction Chemical Equation In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. Titration of an antacid objective: The balanced chemical equation for the reaction of. Antacid Reaction Chemical Equation.
From lessonlibrarysnotter.z13.web.core.windows.net
Identify The Parts Of A Chemical Equation Antacid Reaction Chemical Equation Other antacids, such as alka seltzer, work in similar ways. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. Below, show the mechanism and products for reaction in which. In this experiment, several brands of antacids will be analyzed to determine the number of moles of. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED 2. You can use a net ionic equation to predict whether a Antacid Reaction Chemical Equation In this experiment, you will standardize a solution of base using the analytical technique known as titration. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid. Antacid Reaction Chemical Equation.
From www.youtube.com
D.4 Antacids (SL) YouTube Antacid Reaction Chemical Equation In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment, you will standardize a solution of base using the analytical technique known as titration. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. Below, show the. Antacid Reaction Chemical Equation.
From chemawareness.blogspot.com
Chem Awareness Chemical Reactions Antacid Reaction Chemical Equation Other antacids, such as alka seltzer, work in similar ways. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. The balanced chemical equation for the reaction of the main ingredient of the antacid tablet (caco 3) with the hcl is as follows. In this experiment we will analyze a typical antacid containing caco3,. Antacid Reaction Chemical Equation.
From www.slideshare.net
Antacids Antacid Reaction Chemical Equation In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment, you will standardize a solution of base using the analytical technique known as titration. The balanced chemical equation. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED The table below lists several common antacid brands, their Antacid Reaction Chemical Equation Below, show the mechanism and products for reaction in which. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250 mg of magnesium hydroxide (mg(oh)2), and the label suggests a. In this experiment. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED 2. You can use a net ionic equation to predict whether a Antacid Reaction Chemical Equation In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. In this experiment, several brands of antacids will be analyzed to determine the number of moles of acid neutralized per tablet and the cost. A simple antacid tablet, milk of magnesia, has the following active ingredient, 250. Antacid Reaction Chemical Equation.
From www.numerade.com
SOLVED Write balanced chemical equation for the reaction of your Antacid Reaction Chemical Equation Titration of an antacid objective: In this experiment we will analyze a typical antacid containing caco3, a base which will react with an acid hcl, to neutralize it. In today’s lab you will analyze antacids which are common medications used to neutralize stomach acid. In this experiment, you will standardize a solution of base using the analytical technique known as. Antacid Reaction Chemical Equation.