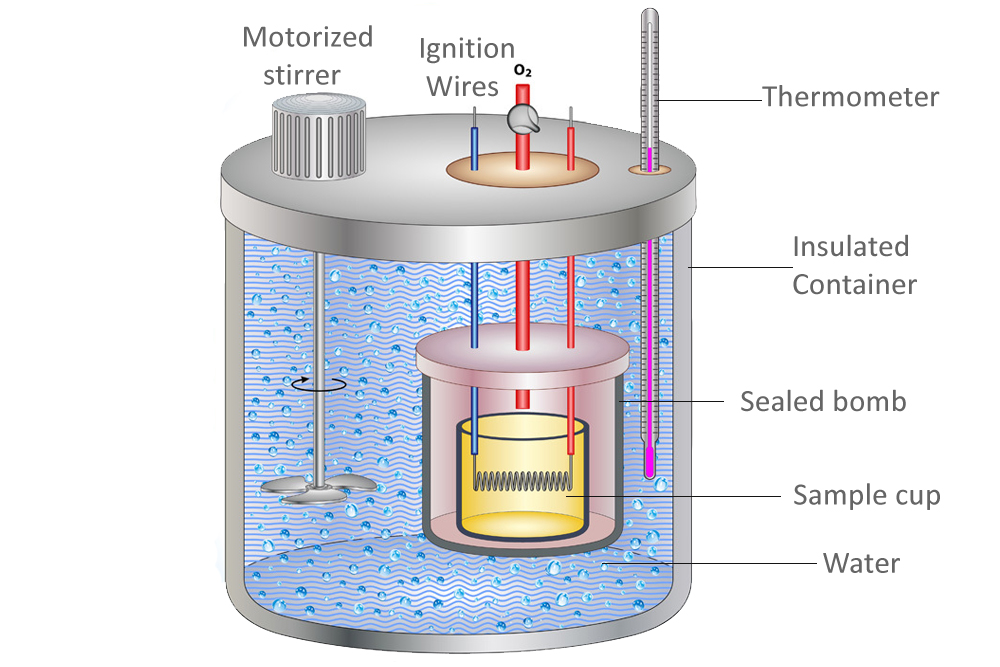
Calorimetry Measures . From the first law we can state. To do so, the heat is exchanged with a calibrated object (calorimeter). Therefore, δesystem = − δesurrounding. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters, which measure. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter.
from www.scienceabc.com
Therefore, δesystem = − δesurrounding. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From the first law we can state. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. It uses devices called calorimeters, which measure. Calorimetry is used to measure amounts of heat transferred to or from a substance.
Molar Heat Capacity Definition, Formula, Equation, Calculation
Calorimetry Measures Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Calorimetry is used to measure amounts of heat transferred to or from a substance. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. From the first law we can state. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Therefore, δesystem = − δesurrounding. To do so, the heat is exchanged with a calibrated object (calorimeter).
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Measures One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a method of measuring the heat transfer within a chemical. Calorimetry Measures.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6655927 Calorimetry Measures Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Calorimetry Measures.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimetry Measures Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is the. Calorimetry Measures.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Measures Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From the first law we can state. Calorimetry is the set of techniques used. Calorimetry Measures.
From studylib.net
Calorimetry Calorimetry Measures Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Therefore, δesystem = − δesurrounding. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use. Calorimetry Measures.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Measures To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetery is. Calorimetry Measures.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Measures One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or. Calorimetry Measures.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Measures Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To do so, the heat is exchanged with a calibrated object (calorimeter). It uses devices called calorimeters, which measure. Therefore, δesystem = − δesurrounding. One technique we can use to measure the amount of heat involved. Calorimetry Measures.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimetry Measures Therefore, δesystem = − δesurrounding. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate. Calorimetry Measures.
From slideplayer.com
Calorimetry CP Unit 9 Chapter ppt download Calorimetry Measures Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes.. Calorimetry Measures.
From slideplayer.com
Calorimetry. ppt download Calorimetry Measures Therefore, δesystem = − δesurrounding. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use. Calorimetry Measures.
From saylordotorg.github.io
Calorimetry Calorimetry Measures Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is. Calorimetry Measures.
From joiddcrwa.blob.core.windows.net
What Is Calorimetry Example at Donovan blog Calorimetry Measures One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It uses devices called calorimeters, which measure. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. From the first. Calorimetry Measures.
From www.roomcalorimeters.com
Understanding Indirect Calorimetry Room Calorimeters Calorimetry Measures Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To do so, the heat is exchanged with a calibrated object (calorimeter). To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the set of. Calorimetry Measures.
From www.youtube.com
Calorimetry calculation YouTube Calorimetry Measures Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is used to measure amounts of heat. Calorimetry Measures.
From saylordotorg.github.io
Calorimetry Calorimetry Measures Therefore, δesystem = − δesurrounding. It uses devices called calorimeters, which measure. Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. From the first law we can state. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used.. Calorimetry Measures.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Measures One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). Therefore, δesystem = − δesurrounding. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Calorimetry Measures.
From slideplayer.com
Calorimetry. ppt download Calorimetry Measures Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter).. Calorimetry Measures.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Measures From the first law we can state. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. To do so, the heat is exchanged with a calibrated object. Calorimetry Measures.
From www.slideserve.com
PPT Differential Scanning Calorimetry PowerPoint Presentation, free Calorimetry Measures One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From the first law we can state. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a. Calorimetry Measures.
From www.slideserve.com
PPT CALORIMETRY PowerPoint Presentation, free download ID5767247 Calorimetry Measures Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Therefore, δesystem = − δesurrounding. It uses devices. Calorimetry Measures.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Measures Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as. Calorimetry Measures.
From www.carolina.com
Food Calorimetry How to Measure Calories in Food Calorimetry Measures To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From the first law we can state. Calorimetry is used to. Calorimetry Measures.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Measures It uses devices called calorimeters, which measure. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. Calorimetry Measures.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Measures To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. To do so, the heat is exchanged with a calibrated object (calorimeter). It uses devices called calorimeters, which measure. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is a method. Calorimetry Measures.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry Measures To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is a method of. Calorimetry Measures.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses Calorimetry Measures Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. It uses devices called calorimeters, which measure. Therefore, δesystem = − δesurrounding. One technique we can use to measure the amount of heat involved in a chemical or physical process is known. Calorimetry Measures.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Measures It uses devices called calorimeters, which measure. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Calorimetery. Calorimetry Measures.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 Calorimetry Measures Therefore, δesystem = − δesurrounding. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. From the first law we. Calorimetry Measures.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Calorimetry Measures Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. From the first law we can state. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Therefore, δesystem. Calorimetry Measures.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Measures Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.. Calorimetry Measures.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Measures To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used. Calorimetry is used to measure amounts of. Calorimetry Measures.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Measures Calorimetry is a method of measuring the heat transfer within a chemical reaction or other physical processes, such as a change between different states of matter. Therefore, δesystem = − δesurrounding. Calorimetry is used to measure amounts of heat transferred to or from a substance. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry is widely used.. Calorimetry Measures.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Measures Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to investigate its physical and chemical changes. Therefore, δesystem = − δesurrounding. One technique we. Calorimetry Measures.
From www.thoughtco.com
Understanding Calorimetry to Measure Heat Transfer Calorimetry Measures One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. It uses devices called calorimeters, which measure. To do so, the heat is exchanged with a calibrated object (calorimeter). From the first law we can state. To determine the enthalpy, stability, heat capacity, and other thermochemical quantities, calorimetry. Calorimetry Measures.