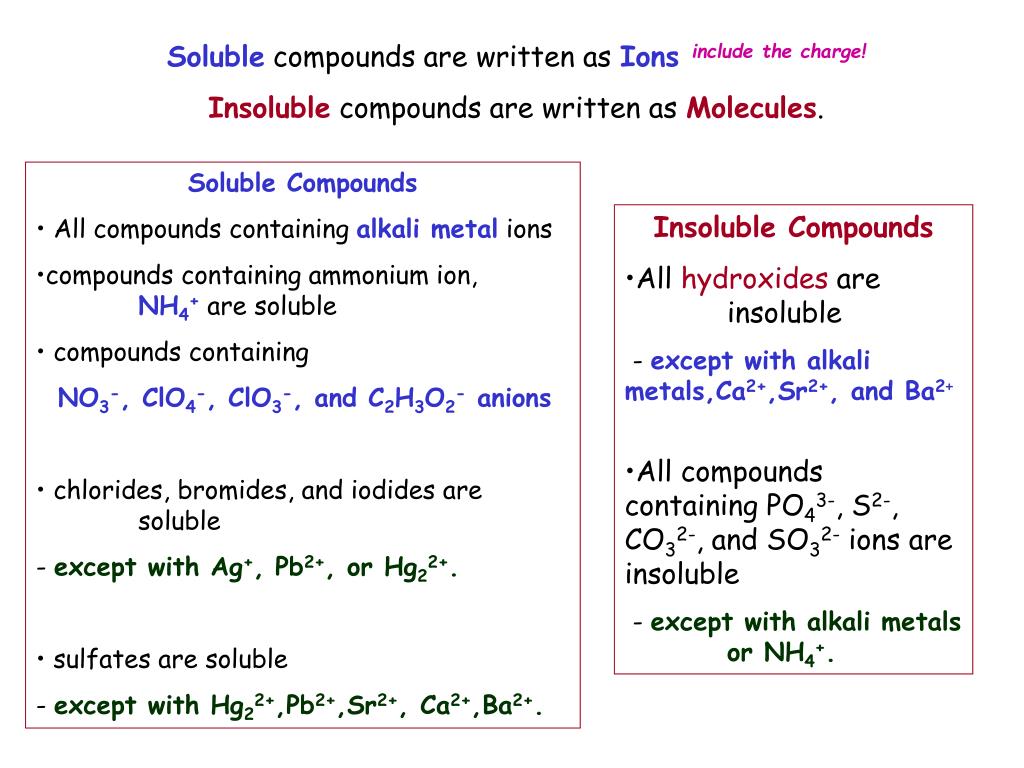
What Ionic Compounds Are Soluble In Water . Salts of the alkali metals, plus nh4 +, are usually soluble. This includes li +, na +, k +, rb +, cs +. The extent to which a substance may be dissolved in water, or any. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Our article on solubility explains how ionic compounds dissolve in water to form a solution. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. So are acetates, chlorates and perchlorates. This process represents a physical change known as dissociation.
from www.slideserve.com
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. Predict solubilities of ionic compounds in water using the solubility rules. This process represents a physical change known as dissociation. This includes li +, na +, k +, rb +, cs +. So are acetates, chlorates and perchlorates. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. The extent to which a substance may be dissolved in water, or any. Salts of the alkali metals, plus nh4 +, are usually soluble.
PPT With enough water molecules, a soluble ionic compound is
What Ionic Compounds Are Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Our article on solubility explains how ionic compounds dissolve in water to form a solution. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. The extent to which a substance may be dissolved in water, or any. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. So are acetates, chlorates and perchlorates. Salts of the alkali metals, plus nh4 +, are usually soluble. This includes li +, na +, k +, rb +, cs +. Predict solubilities of ionic compounds in water using the solubility rules. This process represents a physical change known as dissociation. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID6635190 What Ionic Compounds Are Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Our article on solubility explains how ionic. What Ionic Compounds Are Soluble In Water.
From www.chegg.com
Solved Some solubility rules for ionic compounds in water What Ionic Compounds Are Soluble In Water Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Our article on solubility explains how ionic compounds dissolve in water to form a solution. So are acetates, chlorates and perchlorates. The extent to which a substance. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solution Chemistry PowerPoint Presentation, free download ID What Ionic Compounds Are Soluble In Water Predict solubilities of ionic compounds in water using the solubility rules. So are acetates, chlorates and perchlorates. Our article on solubility explains how ionic compounds dissolve in water to form a solution. This includes li +, na +, k +, rb +, cs +. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. What Ionic Compounds Are Soluble In Water.
From study.com
Compound Solubility in Water Overview & Examples Lesson What Ionic Compounds Are Soluble In Water When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. This includes li +, na +, k +, rb +, cs +. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the. What Ionic Compounds Are Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Ionic Compounds Are Soluble In Water Salts of the alkali metals, plus nh4 +, are usually soluble. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Predict solubilities of ionic compounds in water using the solubility rules. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. The extent to. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solubility Rules & Net Ionic Equations PowerPoint Presentation What Ionic Compounds Are Soluble In Water The extent to which a substance may be dissolved in water, or any. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. So are acetates, chlorates and perchlorates. This process represents a physical change known as dissociation. Our article on solubility explains how ionic compounds dissolve in water to. What Ionic Compounds Are Soluble In Water.
From chem1180.blogspot.com
CHEM 1180 17.1 The Solubility Product Constant What Ionic Compounds Are Soluble In Water The extent to which a substance may be dissolved in water, or any. So are acetates, chlorates and perchlorates. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Predict solubilities of ionic compounds in water using the solubility rules. Salts of the alkali metals, plus nh4 +,. What Ionic Compounds Are Soluble In Water.
From chemcollective.org
CHEM 1315 Lab 10 Conservation of Mass What Ionic Compounds Are Soluble In Water This process represents a physical change known as dissociation. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is What Ionic Compounds Are Soluble In Water So are acetates, chlorates and perchlorates. Predict solubilities of ionic compounds in water using the solubility rules. This process represents a physical change known as dissociation. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. This includes li +, na +, k +, rb +, cs +.. What Ionic Compounds Are Soluble In Water.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry What Ionic Compounds Are Soluble In Water This process represents a physical change known as dissociation. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using. What Ionic Compounds Are Soluble In Water.
From www.pinterest.co.uk
Solubility of ionic solids. Ionic, Solubility, Chemistry What Ionic Compounds Are Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Predict solubilities of ionic compounds in water using the solubility rules. So are acetates, chlorates and perchlorates. When solute mixes with solvent, it can dissolve and form. What Ionic Compounds Are Soluble In Water.
From klanamqvi.blob.core.windows.net
Why Are Ionic Compounds Easily Soluble In Water at Tom Ungar blog What Ionic Compounds Are Soluble In Water This process represents a physical change known as dissociation. Salts of the alkali metals, plus nh4 +, are usually soluble. The extent to which a substance may be dissolved in water, or any. Predict solubilities of ionic compounds in water using the solubility rules. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT UNIT 5 PowerPoint Presentation, free download ID2276550 What Ionic Compounds Are Soluble In Water This process represents a physical change known as dissociation. So are acetates, chlorates and perchlorates. Predict solubilities of ionic compounds in water using the solubility rules. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. When ionic compounds dissolve in water, the ions in the solid separate and disperse. What Ionic Compounds Are Soluble In Water.
From socratic.org
Which of the following substances are insoluble in water? Socratic What Ionic Compounds Are Soluble In Water So are acetates, chlorates and perchlorates. The extent to which a substance may be dissolved in water, or any. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Predict solubilities of ionic compounds in water using the solubility rules. When solute mixes with solvent, it can dissolve. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Chapter 5 Molecular View of Reactions in Aqueous Solutions Part I What Ionic Compounds Are Soluble In Water Salts of the alkali metals, plus nh4 +, are usually soluble. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. The extent to which a substance may be dissolved in water, or any. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout. What Ionic Compounds Are Soluble In Water.
From www.numerade.com
SOLVED Solubility Rulesfor some ionic compounds in water Soluble Ionic What Ionic Compounds Are Soluble In Water Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. The extent to which a substance may be dissolved in water, or any. Our article on solubility explains how ionic compounds dissolve in water to form a. What Ionic Compounds Are Soluble In Water.
From www.sliderbase.com
Properties of Water Presentation Biology What Ionic Compounds Are Soluble In Water Salts of the alkali metals, plus nh4 +, are usually soluble. Predict solubilities of ionic compounds in water using the solubility rules. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. So are acetates, chlorates and perchlorates. Our article on solubility explains how ionic compounds dissolve in water to. What Ionic Compounds Are Soluble In Water.
From www.visionlearning.com
Solutions, Solubility, and Colligative Properties Chemistry What Ionic Compounds Are Soluble In Water Our article on solubility explains how ionic compounds dissolve in water to form a solution. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. The extent to which a substance may be dissolved in water, or any. When solute mixes with solvent, it can dissolve and form. What Ionic Compounds Are Soluble In Water.
From ar.inspiredpencil.com
Solubility In Water What Ionic Compounds Are Soluble In Water Salts of the alkali metals, plus nh4 +, are usually soluble. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Our article on solubility explains how ionic compounds dissolve in water to form a solution. When ionic compounds dissolve in water, the ions in the solid separate. What Ionic Compounds Are Soluble In Water.
From www.youtube.com
Why are ionic compounds soluble in water? YouTube What Ionic Compounds Are Soluble In Water So are acetates, chlorates and perchlorates. Salts of the alkali metals, plus nh4 +, are usually soluble. The extent to which a substance may be dissolved in water, or any. Predict solubilities of ionic compounds in water using the solubility rules. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. What Ionic Compounds Are Soluble In Water.
From wou.edu
CH104 Chapter 7 Solutions Chemistry What Ionic Compounds Are Soluble In Water When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. So are acetates, chlorates and perchlorates. Our article on solubility explains how ionic compounds dissolve in water to form a solution. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Ionic and Covalent bonding PowerPoint Presentation, free download What Ionic Compounds Are Soluble In Water The extent to which a substance may be dissolved in water, or any. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. When ionic compounds dissolve in water, the. What Ionic Compounds Are Soluble In Water.
From www.pinterest.com
Solubility Rules Chart for Chemistry Classroom 11th chemistry What Ionic Compounds Are Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. Salts of the alkali metals,. What Ionic Compounds Are Soluble In Water.
From bceweb.org
Solubility Of Compounds In Water Chart A Visual Reference of Charts What Ionic Compounds Are Soluble In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. This process represents a physical change known. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solubility of Ionic Compounds PowerPoint Presentation, free What Ionic Compounds Are Soluble In Water Salts of the alkali metals, plus nh4 +, are usually soluble. Predict solubilities of ionic compounds in water using the solubility rules. This process represents a physical change known as dissociation. This includes li +, na +, k +, rb +, cs +. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Reaction Stoichiometry Mole Method Calculations PowerPoint What Ionic Compounds Are Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. This includes li +, na +, k +, rb +, cs +. Predict solubilities of ionic compounds in. What Ionic Compounds Are Soluble In Water.
From rayb78.github.io
Solubility In Water Chart What Ionic Compounds Are Soluble In Water So are acetates, chlorates and perchlorates. This includes li +, na +, k +, rb +, cs +. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. Salts of the alkali metals, plus nh4 +, are usually soluble. This process represents a physical change known as dissociation. Predict solubilities. What Ionic Compounds Are Soluble In Water.
From webmis.highland.cc.il.us
Aqueous Solutions What Ionic Compounds Are Soluble In Water Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. Our article on solubility explains how ionic compounds dissolve in water to form a solution. This includes li +, na +, k +, rb +, cs +. Salts of the alkali metals, plus nh4 +, are usually soluble. Predict solubilities. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Chapter 7 Reactions in Aqueous Solutions PowerPoint Presentation What Ionic Compounds Are Soluble In Water Salts of the alkali metals, plus nh4 +, are usually soluble. This process represents a physical change known as dissociation. The extent to which a substance may be dissolved in water, or any. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as a precipitate. This includes li +, na +, k. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solutions & Solubility PowerPoint Presentation, free download What Ionic Compounds Are Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Predict solubilities of ionic compounds in water using the solubility rules. The extent to which a substance may be dissolved in water, or any. When solute mixes with solvent, it can dissolve and form a solution or fall. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID2493612 What Ionic Compounds Are Soluble In Water This process represents a physical change known as dissociation. Our article on solubility explains how ionic compounds dissolve in water to form a solution. This includes li +, na +, k +, rb +, cs +. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate. What Ionic Compounds Are Soluble In Water.
From www.slideserve.com
PPT With enough water molecules, a soluble ionic compound is What Ionic Compounds Are Soluble In Water Salts of the alkali metals, plus nh4 +, are usually soluble. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation. Our article on solubility explains how ionic. What Ionic Compounds Are Soluble In Water.
From klanamqvi.blob.core.windows.net
Why Are Ionic Compounds Easily Soluble In Water at Tom Ungar blog What Ionic Compounds Are Soluble In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. When solute mixes with solvent, it can dissolve and form a solution or fall to the bottom as. What Ionic Compounds Are Soluble In Water.
From shareeducatonideas.com
What Is An Ionic Compound? Formula and Defination What Ionic Compounds Are Soluble In Water So are acetates, chlorates and perchlorates. Our article on solubility explains how ionic compounds dissolve in water to form a solution. This includes li +, na +, k +, rb +, cs +. Review solubility rules for common ionic compounds in water, including calcium carbonate, barium sulfate, and sodium sulfate, using the provided. When ionic compounds dissolve in water, the. What Ionic Compounds Are Soluble In Water.
From www.ck12.org
Physical Properties of Ionic Compounds ( Read ) Chemistry CK12 What Ionic Compounds Are Soluble In Water This includes li +, na +, k +, rb +, cs +. Predict solubilities of ionic compounds in water using the solubility rules. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround. Salts of the alkali metals, plus nh4 +, are usually soluble. The extent to which. What Ionic Compounds Are Soluble In Water.