Threshold Energy Is . Typical values for e th range from 5 to 40 ev and depend on. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the threshold energy, e th, is the minimum energy required to do this. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. For a chemical reaction to occur, an energy. activation energy and threshold energy are both concepts used in chemical reactions. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Activation energy refers to the minimum. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear.
from www.numerade.com
Activation energy refers to the minimum. Typical values for e th range from 5 to 40 ev and depend on. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. activation energy and threshold energy are both concepts used in chemical reactions. For a chemical reaction to occur, an energy. the threshold energy, e th, is the minimum energy required to do this. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two.
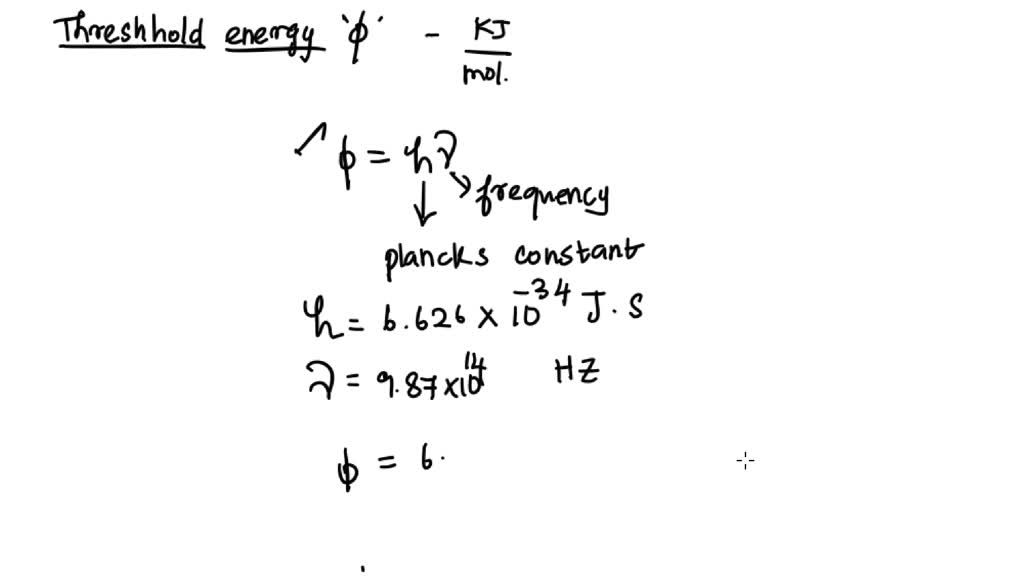
SOLVED Calculate the threshold energy in kJ/mol of electrons in
Threshold Energy Is the threshold energy, e th, is the minimum energy required to do this. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. the threshold energy, e th, is the minimum energy required to do this. For a chemical reaction to occur, an energy. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. Activation energy refers to the minimum. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. activation energy and threshold energy are both concepts used in chemical reactions. Typical values for e th range from 5 to 40 ev and depend on. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur.
From www.researchgate.net
The threshold energy of the СННН process versus temperature for the Threshold Energy Is collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the minimum energy that molecules need to have in order for a reaction to take place is called the. Threshold Energy Is.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy Is Typical values for e th range from 5 to 40 ev and depend on. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. activation. Threshold Energy Is.
From www.researchgate.net
An example of the threshold energy determination for aC sample Threshold Energy Is Activation energy refers to the minimum. activation energy and threshold energy are both concepts used in chemical reactions. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process,. Threshold Energy Is.
From byjus.com
select the incorrect statement (1) The minimum amount of energy Threshold Energy Is the threshold energy, e th, is the minimum energy required to do this. Activation energy refers to the minimum. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. activation energy and threshold energy are both concepts used in chemical reactions. collision theory provides a. Threshold Energy Is.
From giowgpxae.blob.core.windows.net
Threshold Energy Relativity at Jo Foote blog Threshold Energy Is activation energy and threshold energy are both concepts used in chemical reactions. For a chemical reaction to occur, an energy. Activation energy refers to the minimum. the threshold energy, e th, is the minimum energy required to do this. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such. Threshold Energy Is.
From edurev.in
Difference between activation energy and threshold energy I know the Threshold Energy Is collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. activation energy and threshold energy are both concepts used in chemical reactions. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. the minimum energy that molecules need to have in order. Threshold Energy Is.
From www.mdpi.com
Applied Sciences Free FullText MultiDirectional Displacement Threshold Energy Is the threshold energy, e th, is the minimum energy required to do this. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the. Threshold Energy Is.
From www.researchgate.net
(a) As in figure 4(a) but far from the threshold energy region (ω/I Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the threshold energy, e th, is the minimum energy required to do this. For a chemical reaction to occur, an energy. the minimum energy that molecules need to have in order for a reaction to. Threshold Energy Is.
From www.slideserve.com
PPT Radioactivity 29.3 PowerPoint Presentation ID2979284 Threshold Energy Is the threshold energy, e th, is the minimum energy required to do this. activation energy and threshold energy are both concepts used in chemical reactions. Typical values for e th range from 5 to 40 ev and depend on. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. threshold. Threshold Energy Is.
From www.youtube.com
Difference between Activation energy and Threshold energy YouTube Threshold Energy Is collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. the threshold energy, e th, is the minimum energy required to do this. For a chemical reaction to occur, an energy. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a. Threshold Energy Is.
From www.researchgate.net
The threshold energy, √ s th , for K + Λ and K + Σ Download Threshold Energy Is the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. activation energy and threshold energy are both concepts used in chemical reactions. For a chemical reaction to occur, an energy. Typical values for e th range from 5 to 40 ev and depend on. threshold energy. Threshold Energy Is.
From www.differencebetween.com
Difference Between Activation Energy and Threshold Energy Compare the Threshold Energy Is Typical values for e th range from 5 to 40 ev and depend on. the threshold energy, e th, is the minimum energy required to do this. activation energy and threshold energy are both concepts used in chemical reactions. the minimum energy that molecules need to have in order for a reaction to take place is called. Threshold Energy Is.
From scoop.eduncle.com
What is the formula to find q value and threshold energy of a nuclear Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. For a chemical reaction to occur, an energy. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. collision theory provides a qualitative explanation. Threshold Energy Is.
From www.researchgate.net
(a) Energy (Er in a.u.) and the threshold energy (E2s in a.u.) vs. 1 Z Threshold Energy Is collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. the threshold energy, e th, is the minimum energy required to do this. Activation energy refers to the minimum. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. . Threshold Energy Is.
From www.researchgate.net
(PDF) Threshold Energy Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. Typical values for e th range from 5 to 40 ev and depend on. Activation energy refers to the. Threshold Energy Is.
From www.periodic-table.org
What is Critical Energy Threshold Energy for Fission Definition Threshold Energy Is Activation energy refers to the minimum. activation energy and threshold energy are both concepts used in chemical reactions. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process,. Threshold Energy Is.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the threshold energy, e th, is the minimum energy required to do this. Typical values for e th range from 5 to 40 ev and depend on. the minimum energy that molecules need to have. Threshold Energy Is.
From fyorwyame.blob.core.windows.net
Threshold Excitation Energy at Julia White blog Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. For a chemical reaction to occur, an energy. the threshold energy, e th, is the minimum energy required to do this. Activation energy refers to the minimum. threshold energy is the minimum amount of energy required. Threshold Energy Is.
From www.numerade.com
SOLVED Calculate the threshold energy in kJ/mol of electrons in Threshold Energy Is the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. activation energy and threshold energy are both concepts used in chemical reactions. threshold. Threshold Energy Is.
From www.studocu.com
Threshold Energy Threshold Energy Consider the reaction x+X→y+Y We Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. Typical values for e th range from 5 to 40 ev and depend on. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. Activation energy refers to the minimum.. Threshold Energy Is.
From byjus.com
What is threshold frequency and threshold energy? Threshold Energy Is Activation energy refers to the minimum. Typical values for e th range from 5 to 40 ev and depend on. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. the threshold energy, e th, is the minimum energy required to do this. threshold energy is. Threshold Energy Is.
From byjus.com
What happens when the energy of a reaction if more than threshold Threshold Energy Is Typical values for e th range from 5 to 40 ev and depend on. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. activation energy and threshold energy are both concepts used in chemical reactions. Activation energy refers to the minimum. threshold energy is. Threshold Energy Is.
From fyotwgwoo.blob.core.windows.net
Threshold Energy Symbol at Marvin Bly blog Threshold Energy Is Activation energy refers to the minimum. Typical values for e th range from 5 to 40 ev and depend on. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. the minimum energy that molecules need to have in order for a reaction to take place is. Threshold Energy Is.
From giogipcdi.blob.core.windows.net
Threshold Impact Energy at Susan Asbury blog Threshold Energy Is For a chemical reaction to occur, an energy. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. activation energy and threshold energy are. Threshold Energy Is.
From socratic.org
What is activation energy? What is threshold energy? What are the Threshold Energy Is For a chemical reaction to occur, an energy. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to. Threshold Energy Is.
From www.numerade.com
SOLVEDDetermine the Q value and the threshold energy for 8^16 O+ 0^1 Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. the threshold energy, e th, is the minimum energy required to do this. Activation energy refers to the minimum. the minimum energy that molecules need to have in order for a reaction to take place is. Threshold Energy Is.
From byjus.com
26. What is difference between threshold energy and activation energy? Threshold Energy Is threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. the minimum energy that molecules need to have in order for a reaction to take place is. Threshold Energy Is.
From www.youtube.com
, The threshold energy is given as E_0 and radiationof energy E falls Threshold Energy Is activation energy and threshold energy are both concepts used in chemical reactions. the threshold energy, e th, is the minimum energy required to do this. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. collision theory provides a qualitative explanation of chemical reactions and. Threshold Energy Is.
From www.youtube.com
What is activation energy Threshold energy energy barrier rate of Threshold Energy Is For a chemical reaction to occur, an energy. the threshold energy, e th, is the minimum energy required to do this. Typical values for e th range from 5 to 40 ev and depend on. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. the. Threshold Energy Is.
From www.youtube.com
Threshold EnergyEndoergic ReactionsCalculations by Dr. Muhammad Threshold Energy Is threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such as a nuclear. activation energy and threshold energy are both concepts used in chemical reactions. the minimum energy that molecules. Threshold Energy Is.
From www.youtube.com
The threshold wavelength for emission of electrons from a given metal Threshold Energy Is Activation energy refers to the minimum. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. collision theory provides a qualitative explanation of chemical reactions and the rates at which they occur. threshold energy is the minimum kinetic energy the molecules must have to bring about. Threshold Energy Is.
From www.slideserve.com
PPT General Physics (PHY 2140) PowerPoint Presentation, free download Threshold Energy Is Typical values for e th range from 5 to 40 ev and depend on. threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. For a chemical reaction to occur, an energy. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction or process, such. Threshold Energy Is.
From giowgpxae.blob.core.windows.net
Threshold Energy Relativity at Jo Foote blog Threshold Energy Is threshold energy is the minimum kinetic energy the molecules must have to bring about effective collisions between two. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. the minimum energy that molecules need to have in order for a reaction to take place is. Threshold Energy Is.
From www.diplomageeks.com
Define Threshold frequency,Threshold wavelength,Work function, Stopping Threshold Energy Is activation energy and threshold energy are both concepts used in chemical reactions. For a chemical reaction to occur, an energy. threshold energy is the minimum amount of energy required to initiate a specific process, such as the excitation of an electron from. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction. Threshold Energy Is.
From www.youtube.com
Calculate threshold frequency Dual nature of light Physics Khan Threshold Energy Is Typical values for e th range from 5 to 40 ev and depend on. For a chemical reaction to occur, an energy. the minimum energy that molecules need to have in order for a reaction to take place is called the threshold energy. threshold energy is the minimum amount of energy required to initiate a specific nuclear reaction. Threshold Energy Is.