Calorimetry Lab Worksheet . A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to determine heat flow during a chemical or physical change. If the combustion of 0.175 moles. How much energy is needed to change the temperature of 50.0 g of water by. Calorimetry problems 1 solve the following problems. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. As always, include work and show the units to ensure full credit. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. As you work through the steps in the lab procedures, record your experimental values and the results on this. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. In this lab, you will do two classic calorimetry experiments:
from www.studocu.com
How much energy is needed to change the temperature of 50.0 g of water by. A calorimeter is a device used to determine heat flow during a chemical or physical change. If the combustion of 0.175 moles. A doubled styrofoam cup fitted with a cover in. As you work through the steps in the lab procedures, record your experimental values and the results on this. In this lab, you will do two classic calorimetry experiments: As always, include work and show the units to ensure full credit. Calorimetry problems 1 solve the following problems. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,.
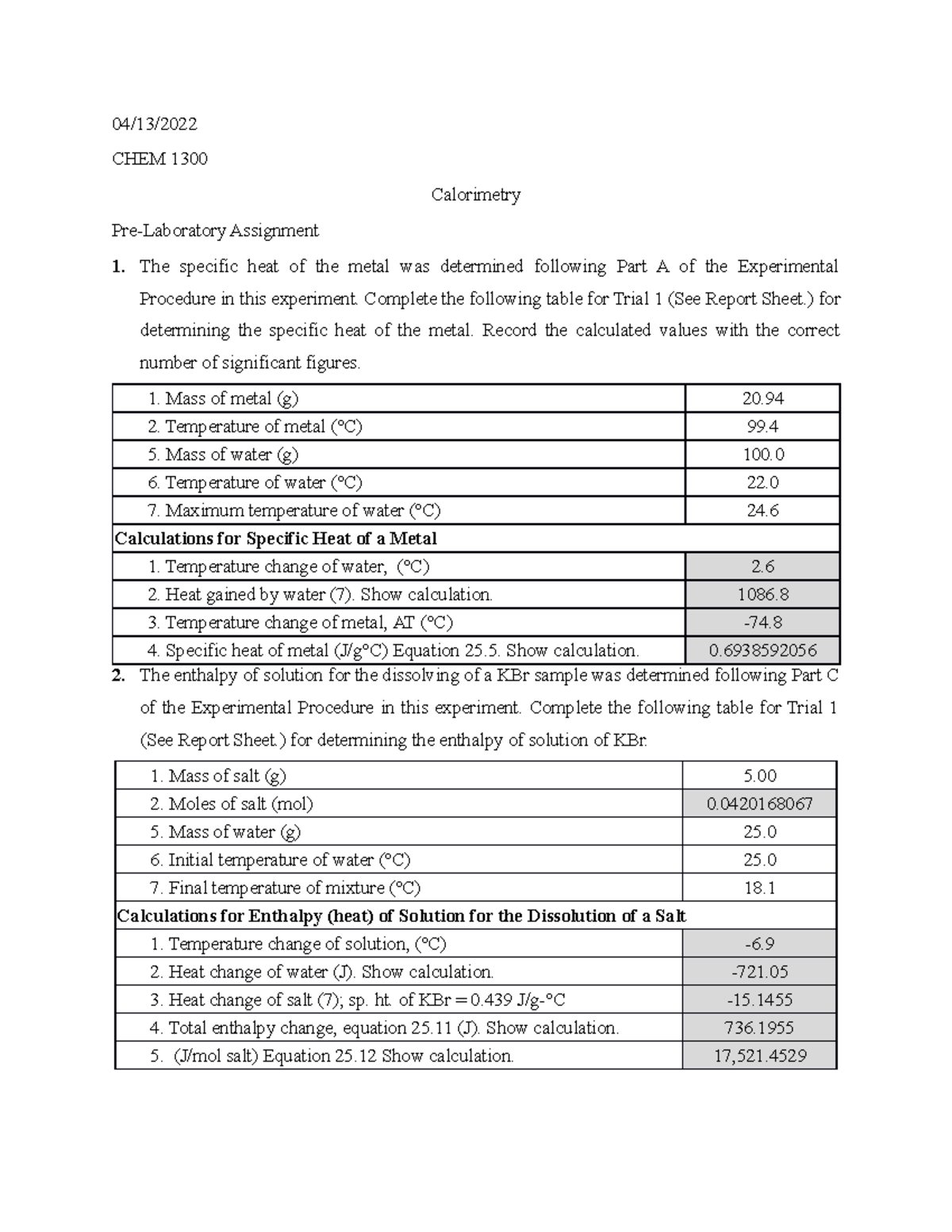
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory
Calorimetry Lab Worksheet A doubled styrofoam cup fitted with a cover in. Calorimetry problems 1 solve the following problems. A doubled styrofoam cup fitted with a cover in. How much energy is needed to change the temperature of 50.0 g of water by. As you work through the steps in the lab procedures, record your experimental values and the results on this. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. As always, include work and show the units to ensure full credit. A calorimeter is a device used to determine heat flow during a chemical or physical change. If the combustion of 0.175 moles. In this lab, you will do two classic calorimetry experiments:
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Lab Worksheet If the combustion of 0.175 moles. As you work through the steps in the lab procedures, record your experimental values and the results on this. How much energy is needed to change the temperature of 50.0 g of water by. In this lab, you will do two classic calorimetry experiments: A doubled styrofoam cup fitted with a cover in. Measuring. Calorimetry Lab Worksheet.
From gonaalone.blogspot.com
Calorimetry Worksheet Answers Chapter 5 Thermochemistry / Back to Calorimetry Lab Worksheet A doubled styrofoam cup fitted with a cover in. In this lab, you will do two classic calorimetry experiments: As you work through the steps in the lab procedures, record your experimental values and the results on this. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. In this set of. Calorimetry Lab Worksheet.
From www.studocu.com
Dry ABC DRY LAB A BOMB CALORIMETRY WORKSHEET A l Work Sheet Do Calorimetry Lab Worksheet Calorimetry problems 1 solve the following problems. As you work through the steps in the lab procedures, record your experimental values and the results on this. As always, include work and show the units to ensure full credit. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. In this lab, you. Calorimetry Lab Worksheet.
From learningzonebeth.z13.web.core.windows.net
Specific Heat And Calorimetry Worksheets Calorimetry Lab Worksheet Calorimetry problems 1 solve the following problems. A doubled styrofoam cup fitted with a cover in. As you work through the steps in the lab procedures, record your experimental values and the results on this. How much energy is needed to change the temperature of 50.0 g of water by. Compound a is burned in a bomb calorimeter that contains. Calorimetry Lab Worksheet.
From www.chegg.com
Solved Chapter 6Calorimetry Worksheet Key Open the Calorimetry Lab Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Calorimetry problems 1 solve the following problems. If the combustion of 0.175 moles. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. In this set of practice questions, we will go over the main types of questions. Calorimetry Lab Worksheet.
From jiahui-fahrenheitnanime.blogspot.com
Calorimetry Worksheet Calorimetry Lab Worksheet As always, include work and show the units to ensure full credit. If the combustion of 0.175 moles. Calorimetry problems 1 solve the following problems. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity,. Calorimetry Lab Worksheet.
From chem.libretexts.org
2.7 Calorimetry Chemistry LibreTexts Calorimetry Lab Worksheet A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to determine heat flow during a chemical or physical change. How much energy is needed to change the temperature of 50.0 g of water by. As always, include work and show the units to ensure full credit. If the combustion of 0.175 moles. In this. Calorimetry Lab Worksheet.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Lab Worksheet How much energy is needed to change the temperature of 50.0 g of water by. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. If the combustion of 0.175 moles. Calorimetry problems 1 solve the following problems. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water.. Calorimetry Lab Worksheet.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Lab Worksheet In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. If the combustion of 0.175 moles. A doubled styrofoam cup fitted with a cover in. How much energy is needed to change the temperature of 50.0 g of water by. Measuring the latent heat of. Calorimetry Lab Worksheet.
From www.worksheeto.com
18 Best Images of For Specific Heat Worksheet Physics Energy Phase Calorimetry Lab Worksheet Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. If the combustion of 0.175 moles. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. As always, include work and show the units to ensure full. Calorimetry Lab Worksheet.
From gonaalone.blogspot.com
Calorimetry Worksheet Answers Chapter 5 Thermochemistry / Back to Calorimetry Lab Worksheet A calorimeter is a device used to determine heat flow during a chemical or physical change. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. How much energy is needed to change the temperature of 50.0 g of water by. As you work through the steps in the lab procedures, record your experimental values and. Calorimetry Lab Worksheet.
From printableschoolesenhar.z21.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Lab Worksheet A doubled styrofoam cup fitted with a cover in. A calorimeter is a device used to determine heat flow during a chemical or physical change. As you work through the steps in the lab procedures, record your experimental values and the results on this. In this set of practice questions, we will go over the main types of questions on. Calorimetry Lab Worksheet.
From chessmuseum.org
50 Calorimetry Worksheet Answer Key Calorimetry Lab Worksheet Calorimetry problems 1 solve the following problems. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. As you work through the steps in the lab procedures, record your experimental values and the results on this. If the combustion of 0.175 moles. A calorimeter is a device used to determine heat flow. Calorimetry Lab Worksheet.
From sites.google.com
3.2.1 (e) Q=mcΔT Ellesmere OCR A level Chemistry Calorimetry Lab Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. As always, include work and show the units to ensure full credit. In this lab, you will do two classic calorimetry experiments: If the combustion of 0.175 moles. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals.. Calorimetry Lab Worksheet.
From answerschoolkane101.z21.web.core.windows.net
Calorimetry Gizmo Worksheet Answers Calorimetry Lab Worksheet A calorimeter is a device used to determine heat flow during a chemical or physical change. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. How much energy is needed to change the temperature of 50.0 g of water by. A doubled styrofoam cup fitted with a cover in. In this set of practice questions,. Calorimetry Lab Worksheet.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Calorimetry Lab Worksheet A doubled styrofoam cup fitted with a cover in. As you work through the steps in the lab procedures, record your experimental values and the results on this. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. In this lab, you will do two classic calorimetry experiments: How much energy is. Calorimetry Lab Worksheet.
From www.pinterest.jp
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library Calorimetry Lab Worksheet A calorimeter is a device used to determine heat flow during a chemical or physical change. Calorimetry problems 1 solve the following problems. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. If the combustion of 0.175 moles. In this lab, you will do. Calorimetry Lab Worksheet.
From studylib.net
Calorimetry Lab Report Problem To experimentally determine the Calorimetry Lab Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. A calorimeter is a device used to determine heat flow during a chemical or physical change. If the combustion of 0.175 moles. In this lab, you will do two classic calorimetry experiments: As you work through the steps in the lab procedures, record your experimental values. Calorimetry Lab Worksheet.
From www.pinterest.co.uk
Pin on Science Friday Calorimetry Lab Worksheet How much energy is needed to change the temperature of 50.0 g of water by. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. In this lab, you will do two classic calorimetry experiments: A calorimeter is a device used to determine heat flow during a chemical or physical change. If the combustion of 0.175. Calorimetry Lab Worksheet.
From printablefulltipsy.z13.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Lab Worksheet Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. As always, include work and show the units to ensure full credit. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. A calorimeter is a device. Calorimetry Lab Worksheet.
From zipworksheet.com
Calorimetry Worksheet Answer Key Calorimetry Lab Worksheet In this lab, you will do two classic calorimetry experiments: As always, include work and show the units to ensure full credit. As you work through the steps in the lab procedures, record your experimental values and the results on this. In this set of practice questions, we will go over the main types of questions on calorimetry including the. Calorimetry Lab Worksheet.
From studylib.net
Calorimetry Worksheet Calorimetry Lab Worksheet Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. How much energy is needed to change the temperature of 50.0 g of water by. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Compound a. Calorimetry Lab Worksheet.
From www.studocu.com
Worksheet Chapter 6 calorimetry Key CHEM 1300 General Chemistry I Calorimetry Lab Worksheet If the combustion of 0.175 moles. Calorimetry problems 1 solve the following problems. As you work through the steps in the lab procedures, record your experimental values and the results on this. As always, include work and show the units to ensure full credit. A calorimeter is a device used to determine heat flow during a chemical or physical change.. Calorimetry Lab Worksheet.
From studylib.net
Cheeto Calorimetry Lab Calorimetry Lab Worksheet As you work through the steps in the lab procedures, record your experimental values and the results on this. Calorimetry problems 1 solve the following problems. A calorimeter is a device used to determine heat flow during a chemical or physical change. If the combustion of 0.175 moles. How much energy is needed to change the temperature of 50.0 g. Calorimetry Lab Worksheet.
From learningzonenujenul.z14.web.core.windows.net
Heat And Calorimetry Worksheets Calorimetry Lab Worksheet How much energy is needed to change the temperature of 50.0 g of water by. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Calorimetry problems 1 solve the following problems. If the combustion of 0.175 moles. Measuring the latent heat of fusion of. Calorimetry Lab Worksheet.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Lab Worksheet How much energy is needed to change the temperature of 50.0 g of water by. Calorimetry problems 1 solve the following problems. A calorimeter is a device used to determine heat flow during a chemical or physical change. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the. Calorimetry Lab Worksheet.
From www.vrogue.co
Calorimetry Worksheet Answer Key New Calorimetry Work vrogue.co Calorimetry Lab Worksheet As you work through the steps in the lab procedures, record your experimental values and the results on this. Calorimetry problems 1 solve the following problems. As always, include work and show the units to ensure full credit. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the. Calorimetry Lab Worksheet.
From www.studocu.com
Gizmo Calorimetry Lab worksheet Name Calorimetry Lab Worksheet A calorimeter is a device used to determine heat flow during a chemical or physical change. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. A doubled styrofoam cup fitted with a cover in. How much energy is needed to change the temperature of 50.0 g of water by. If the. Calorimetry Lab Worksheet.
From www.chegg.com
Solved Chapter 6 Calorimetry Worksheet Key Open the Calorimetry Lab Worksheet Calorimetry problems 1 solve the following problems. As you work through the steps in the lab procedures, record your experimental values and the results on this. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat. Calorimetry Lab Worksheet.
From studylib.net
Lab 33 calorimetry lab Calorimetry Lab Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. A calorimeter is a device used to determine heat flow during a chemical or physical change. A doubled styrofoam cup fitted with a cover in. If the combustion of 0.175 moles. In this set of practice questions, we will go over the main types of questions. Calorimetry Lab Worksheet.
From www.artofit.org
Calorimetry worksheet answer key elegant virtual calorimeter lab Calorimetry Lab Worksheet In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. As you work through the steps in the lab procedures, record your experimental values and the results on this. Calorimetry problems 1 solve the following problems. If the combustion of 0.175 moles. Measuring the latent. Calorimetry Lab Worksheet.
From www.chegg.com
Solved Student Exploration Calorimetry Lab Vocabulary Calorimetry Lab Worksheet Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. If the combustion of 0.175 moles. Calorimetry problems 1 solve the following problems. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. As always, include work and show the units. Calorimetry Lab Worksheet.
From users.highland.edu
Calorimetry Calorimetry Lab Worksheet Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. As you work through the steps in the lab procedures, record your experimental values and the results on this. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat. Calorimetry Lab Worksheet.
From afashionlista.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C / Explore Learning Calorimetry Lab Worksheet A doubled styrofoam cup fitted with a cover in. Compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Measuring the latent heat of fusion of water, and measuring the specific heat capacities of two different metals. If the combustion of 0.175 moles. How much energy is needed to change the temperature of 50.0 g of. Calorimetry Lab Worksheet.
From studylib.net
Calorimetry Worksheet Calorimetry Lab Worksheet As you work through the steps in the lab procedures, record your experimental values and the results on this. In this set of practice questions, we will go over the main types of questions on calorimetry including the heat capacity, the heat of reaction,. Calorimetry problems 1 solve the following problems. Measuring the latent heat of fusion of water, and. Calorimetry Lab Worksheet.