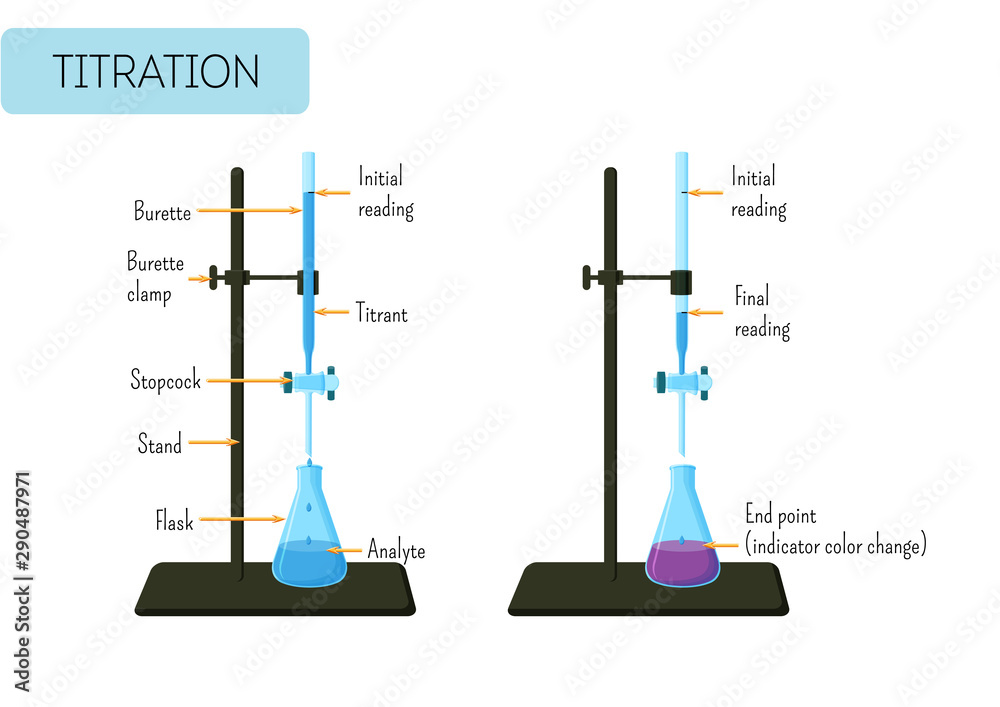
Titration Burette Experiment . learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. a titration experiment is performed when we wish to determine the concentration of an acid or a base. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. The analyte (titrand) is the solution with an unknown molarity. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. running a titration experiment. This video by the royal society of chemistry. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use with different classes, and look at the need for. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The reagent (titrant) is the solution with a known molarity that will react with the analyte.
from hxeevvgwj.blob.core.windows.net
The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. a titration experiment is performed when we wish to determine the concentration of an acid or a base. You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals.
Volumetric Flask And Burette at Julie Christenson blog
Titration Burette Experiment They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. This video by the royal society of chemistry. a titration experiment is performed when we wish to determine the concentration of an acid or a base. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You have to decide if this experiment is suitable to use with different classes, and look at the need for. The analyte (titrand) is the solution with an unknown molarity. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. running a titration experiment. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to Titration Burette Experiment You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. in this experiment students neutralise sodium. Titration Burette Experiment.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Burette Experiment The reagent (titrant) is the solution with a known molarity that will react with the analyte. This video by the royal society of chemistry. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. learn about the titration technique through a practical experiment to determine the reacting volumes. Titration Burette Experiment.
From sbl1023.blogspot.com
LAB 3 TITRATION Titration Burette Experiment The reagent (titrant) is the solution with a known molarity that will react with the analyte. You have to decide if this experiment is suitable to use with different classes, and look at the need for. running a titration experiment. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to. Titration Burette Experiment.
From webstockreview.net
Experiment clipart titration, Experiment titration Transparent FREE for Titration Burette Experiment The analyte (titrand) is the solution with an unknown molarity. running a titration experiment. This video by the royal society of chemistry. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt. Titration Burette Experiment.
From fyoapfhcs.blob.core.windows.net
Titration Lab Simple at Justin Funk blog Titration Burette Experiment You have to decide if this experiment is suitable to use with different classes, and look at the need for. running a titration experiment. a titration experiment is performed when we wish to determine the concentration of an acid or a base. a buret is primarily used for titration to determine the concentration of an unknown solution. Titration Burette Experiment.
From www.directindustry.com
Titration burette 9695 series Bürkle borosilicate glass Titration Burette Experiment This video by the royal society of chemistry. The reagent (titrant) is the solution with a known molarity that will react with the analyte. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. learn. Titration Burette Experiment.
From www.dreamstime.com
Redox Titration with Burette and Pipette Stock Vector Illustration of Titration Burette Experiment to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. This video by the royal society of chemistry. a. Titration Burette Experiment.
From stock.adobe.com
Titration Experiment (Burette and Flask for Titration) simple Titration Burette Experiment running a titration experiment. The analyte (titrand) is the solution with an unknown molarity. You have to decide if this experiment is suitable to use with different classes, and look at the need for. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. a titration experiment is performed when. Titration Burette Experiment.
From www.shalom-education.com
Required Practical Titration with a Strong Acid and a Strong Alkali Titration Burette Experiment 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The reagent. Titration Burette Experiment.
From www.walmart.com
Titration Lab Kit 50ml Burette, 23" (60cm) Rod, 8"x5" Heavy Base Titration Burette Experiment This video by the royal society of chemistry. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. You have to decide if this experiment is suitable to use with different classes, and look. Titration Burette Experiment.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to Titration Burette Experiment learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. a titration experiment is performed when we wish to determine the concentration of an acid or a base. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The reagent (titrant) is the solution with a. Titration Burette Experiment.
From www.istockphoto.com
870+ Titulação fotos de stock, imagens e fotos royaltyfree iStock Titration Burette Experiment learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. . Titration Burette Experiment.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Burette Experiment running a titration experiment. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. to perform a titration, you'll need a calibrated burette, a burette stand,. Titration Burette Experiment.
From resource.studiaacademy.com
edexcel_igcse_chemistry_topic15_acidsalkalisandtitrations_004 Titration Burette Experiment a titration experiment is performed when we wish to determine the concentration of an acid or a base. You have to decide if this experiment is suitable to use with different classes, and look at the need for. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. a buret is primarily used. Titration Burette Experiment.
From techblog.ctgclean.com
Chemistry What is Titration? CTG Clean Titration Burette Experiment You have to decide if this experiment is suitable to use with different classes, and look at the need for. The analyte (titrand) is the solution with an unknown molarity. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a buret is primarily used for titration to determine the concentration of an. Titration Burette Experiment.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to Titration Burette Experiment 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. The reagent (titrant) is the solution with a known molarity that will react with the analyte. You have to decide if this experiment is suitable to use with different classes, and look at the need for. a. Titration Burette Experiment.
From hxeevvgwj.blob.core.windows.net
Volumetric Flask And Burette at Julie Christenson blog Titration Burette Experiment You have to decide if this experiment is suitable to use with different classes, and look at the need for. The analyte (titrand) is the solution with an unknown molarity. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. a titration experiment is performed when we. Titration Burette Experiment.
From www.savemyexams.co.uk
AcidAlkali Titrations (2.6.3) Edexcel IGCSE Chemistry Revision Notes Titration Burette Experiment running a titration experiment. a titration experiment is performed when we wish to determine the concentration of an acid or a base. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks,. Titration Burette Experiment.
From pixels.com
Titration Experiment Photograph by Titration Burette Experiment in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is the solution with an unknown molarity. a buret is primarily used for titration to determine the concentration of an. Titration Burette Experiment.
From pharmaceuticaleducation.blogspot.com
Pharma gyan Pandit Titration Burette Experiment The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. The analyte (titrand) is the solution with an unknown. Titration Burette Experiment.
From www.savemyexams.com
AcidBase Titrations CIE IGCSE Chemistry Revision Notes 2023 Titration Burette Experiment a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. running a titration experiment. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. This video by the royal society of chemistry. to perform a titration, you'll need a calibrated burette, a. Titration Burette Experiment.
From www.amazon.com
Titration Equipment Set Complete Single Buret Burete Assembly with 100 Titration Burette Experiment a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. The reagent (titrant) is the solution with a known molarity that will react with the analyte. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. 1.1 a titration. Titration Burette Experiment.
From www.alamy.com
Titration experiment. Student using a burette and conical flask to Titration Burette Experiment running a titration experiment. a titration experiment is performed when we wish to determine the concentration of an acid or a base. The analyte (titrand) is the solution with an unknown molarity. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. in this experiment students neutralise sodium hydroxide with hydrochloric acid. Titration Burette Experiment.
From fyoxsyypa.blob.core.windows.net
In Titration What Goes In Burette Acid Or Base at Douglas Cox blog Titration Burette Experiment to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. a titration experiment is performed when we wish to determine the concentration of an acid or a base. This video. Titration Burette Experiment.
From edu.rsc.org
Titrating sodium hydroxide with hydrochloric acid Experiment RSC Titration Burette Experiment This video by the royal society of chemistry. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. running a titration experiment. a buret is primarily used for titration to determine the concentration of an. Titration Burette Experiment.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration Burette Experiment learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. The analyte (titrand) is the solution with an unknown molarity. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant) that is required to react completely with. to perform a titration, you'll need a calibrated burette,. Titration Burette Experiment.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Titration Burette Experiment The reagent (titrant) is the solution with a known molarity that will react with the analyte. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. The analyte (titrand) is the solution with an unknown molarity. 1.1 a titration involves measuring the exact volume of a reagent solution (the titrant). Titration Burette Experiment.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Titration Burette Experiment The analyte (titrand) is the solution with an unknown molarity. a titration experiment is performed when we wish to determine the concentration of an acid or a base. This video by the royal society of chemistry. The reagent (titrant) is the solution with a known molarity that will react with the analyte. They then concentrate the solution and allow. Titration Burette Experiment.
From cartoondealer.com
Laboratory Experiment Of Acid Base Titration With Glass Burette And Titration Burette Experiment This video by the royal society of chemistry. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. 1.1 a titration involves measuring the exact volume of a reagent solution (the. Titration Burette Experiment.
From www.alamy.com
Titration experiment. Student taking a volume reading from a burette Titration Burette Experiment learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. You have to decide if this experiment is suitable to use with different classes, and look at the need for. The reagent (titrant) is the solution with a known molarity that will react with the analyte. a buret is primarily used. Titration Burette Experiment.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Burette Experiment a titration experiment is performed when we wish to determine the concentration of an acid or a base. running a titration experiment. a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers. Titration Burette Experiment.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Titration Burette Experiment a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. a titration experiment is performed when we wish to determine the concentration of an acid or a base. The reagent (titrant) is the solution with a known molarity that will react with the analyte. The analyte (titrand) is. Titration Burette Experiment.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Burette Experiment a buret is primarily used for titration to determine the concentration of an unknown solution by adding a solution of. You have to decide if this experiment is suitable to use with different classes, and look at the need for. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. . Titration Burette Experiment.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Burette Experiment The analyte (titrand) is the solution with an unknown molarity. to perform a titration, you'll need a calibrated burette, a burette stand, multiple beakers or erlenmeyer flasks, a. They then concentrate the solution and allow it to crystallise to produce sodium chloride crystals. The reagent (titrant) is the solution with a known molarity that will react with the analyte.. Titration Burette Experiment.
From www.sks-science.com
SKS Science Products Burettes, Automatic Titration Burette Titration Burette Experiment The analyte (titrand) is the solution with an unknown molarity. learn about the titration technique through a practical experiment to determine the reacting volumes and concentration of. This video by the royal society of chemistry. a titration experiment is performed when we wish to determine the concentration of an acid or a base. You have to decide if. Titration Burette Experiment.