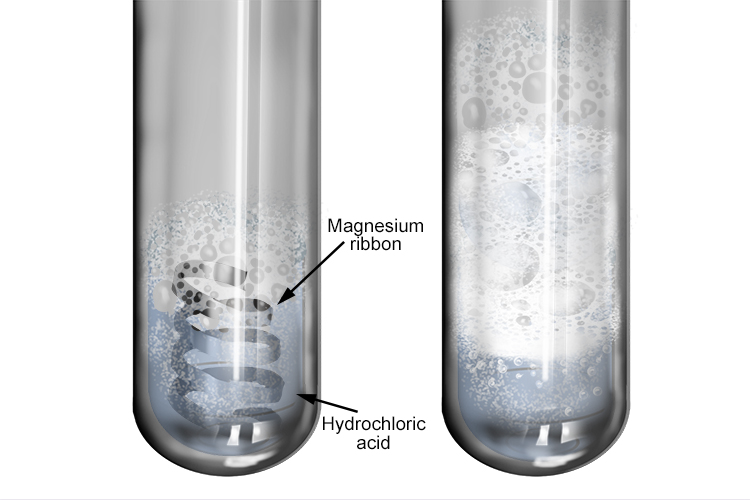
Magnesium Carbonate And Hcl . Includes kit list and safety instructions. The web page explains the reactions, gives examples and shows diagrams and equations. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. The balanced chemical equation is: Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction.
from mammothmemory.net
Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. The web page explains the reactions, gives examples and shows diagrams and equations. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Includes kit list and safety instructions. The balanced chemical equation is: Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts.
Similarly magnesium and hydrochloric acid is a slow reaction
Magnesium Carbonate And Hcl When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. The web page explains the reactions, gives examples and shows diagrams and equations. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. The balanced chemical equation is: Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. Includes kit list and safety instructions. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction.
From studylib.net
magnesium carbonate lab Magnesium Carbonate And Hcl The web page explains the reactions, gives examples and shows diagrams and equations. Includes kit list and safety instructions. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how magnesium carbonate. Magnesium Carbonate And Hcl.
From delantalesybanderines.blogspot.com
Mgco3+Hcl=Mgcl2+H2O+Co2 Ionic Equation delantalesybanderines Magnesium Carbonate And Hcl Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. The balanced chemical equation is: The web page explains the reactions, gives examples and shows diagrams and equations. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) +. Magnesium Carbonate And Hcl.
From www.youtube.com
Magnesium and Hydrochloric Acid Lab YouTube Magnesium Carbonate And Hcl Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. The web page explains the reactions, gives examples and shows diagrams and equations. Includes kit list and safety instructions. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. The balanced chemical equation is: A class practical on reacting magnesium with hydrochloric acid and how to measure. Magnesium Carbonate And Hcl.
From complianceportal.american.edu
💋 Rate of reaction of magnesium with hydrochloric acid. Rate of Magnesium Carbonate And Hcl Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. Includes kit list and safety instructions. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. A. Magnesium Carbonate And Hcl.
From koltenfersrubio.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate And Hcl Includes kit list and safety instructions. The web page explains the reactions, gives examples and shows diagrams and equations. The balanced chemical equation is: Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. When equal. Magnesium Carbonate And Hcl.
From www.youtube.com
How to Write the Net Ionic Equation for HCl + MgCO3 = MgCl2 + CO2 + H2O Magnesium Carbonate And Hcl Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. The balanced chemical equation is: When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide,. Magnesium Carbonate And Hcl.
From www.vrogue.co
Sodium Carbonate Reacts With Hydrochloric Acid Balanc vrogue.co Magnesium Carbonate And Hcl Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. Includes kit list and. Magnesium Carbonate And Hcl.
From fineartamerica.com
Magnesium Carbonate In Hydrochloric Acid Photograph by Martyn F. Chillmaid Magnesium Carbonate And Hcl Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. The balanced chemical equation is: Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Includes kit list and. Magnesium Carbonate And Hcl.
From sak-laop.blogspot.com
Magnesium And Hydrochloric Acid The rate of reaction of magnesium Magnesium Carbonate And Hcl A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g). Magnesium Carbonate And Hcl.
From naturalcalm.ca
Magnesium Carbonate Your Guide to This Type of Magnesium Supplement Magnesium Carbonate And Hcl Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride. Magnesium Carbonate And Hcl.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate And Hcl A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting. Magnesium Carbonate And Hcl.
From sciencekitstore.com
Magnesium Carbonate, Basic, USP, "Extra Light" Magnesium Carbonate And Hcl Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. The web page explains the. Magnesium Carbonate And Hcl.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate And Hcl Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. The web page explains the reactions, gives examples and shows diagrams and equations. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. When equal amounts of a strong acid such as hydrochloric acid are mixed. Magnesium Carbonate And Hcl.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Magnesium Carbonate And Hcl Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Includes kit list and safety instructions. When equal amounts of. Magnesium Carbonate And Hcl.
From www.numerade.com
SOLVED A 15.9 g15.9 g sample of a mixture of magnesium carbonate and Magnesium Carbonate And Hcl Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. The balanced chemical equation is: A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. The web page explains the reactions, gives examples and shows diagrams and equations. Learn how acids react with. Magnesium Carbonate And Hcl.
From linativewise.blogspot.com
Calcium Carbonate and Hydrochloric Acid Magnesium Carbonate And Hcl Includes kit list and safety instructions. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. The balanced chemical equation is: Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Learn how to perform. Magnesium Carbonate And Hcl.
From mungfali.com
PPT Section 9.1 Naming Ions PowerPoint Presentation Magnesium Carbonate And Hcl Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)). Magnesium Carbonate And Hcl.
From mammothmemory.net
Similarly magnesium and hydrochloric acid is a slow reaction Magnesium Carbonate And Hcl A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and.. Magnesium Carbonate And Hcl.
From www.coursehero.com
[Solved] 5. when solid magnesium reacts with aqueous hydrochloric acid Magnesium Carbonate And Hcl The balanced chemical equation is: Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. The web page explains the reactions, gives examples and shows diagrams and equations. When equal amounts of a strong acid such as hydrochloric acid are mixed with. Magnesium Carbonate And Hcl.
From www.chegg.com
Solved A 18.7 g sample of a mixture of magnesium carbonate Magnesium Carbonate And Hcl Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. The balanced chemical equation is: Includes kit list and safety. Magnesium Carbonate And Hcl.
From www.youtube.com
Neutralization of dilute HCl by magnesium carbonate light (MCL) YouTube Magnesium Carbonate And Hcl The balanced chemical equation is: Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. The web page explains the reactions, gives examples and shows diagrams and equations. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. When equal amounts of a strong acid such as. Magnesium Carbonate And Hcl.
From mungfali.com
Magnesium Carbonate Hydrochloric Acid Magnesium Carbonate And Hcl The balanced chemical equation is: A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Includes kit list and safety. Magnesium Carbonate And Hcl.
From www.toppr.com
20.5g of a sample of magnesium carbonate on treatment with excess of Magnesium Carbonate And Hcl The balanced chemical equation is: Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2). Magnesium Carbonate And Hcl.
From mavink.com
Magnesium And Hcl Reaction Magnesium Carbonate And Hcl Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. The web page explains the reactions, gives examples and shows diagrams and equations. Includes kit list and safety instructions. Learn. Magnesium Carbonate And Hcl.
From www.numerade.com
SOLVEDThe mineral dolomite contains magnesium carbonate. MgCO3(s)+2 Magnesium Carbonate And Hcl A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. The balanced chemical equation is: Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such. Magnesium Carbonate And Hcl.
From www.sciencephoto.com
Magnesium carbonate in hydrochloric acid Stock Image A500/0601 Magnesium Carbonate And Hcl Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. The web page explains the reactions, gives examples and shows diagrams and. Magnesium Carbonate And Hcl.
From www.degruyter.com
Synthesis of magnesium carbonate hydrate from natural talc Magnesium Carbonate And Hcl A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. The balanced chemical equation is: When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium. Magnesium Carbonate And Hcl.
From www.numerade.com
SOLVEDA 6.53 g sample of a mixture of magnesium carbonate and calcium Magnesium Carbonate And Hcl Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. The web page explains the reactions, gives examples and shows diagrams and equations. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of. Magnesium Carbonate And Hcl.
From vanspa.vn
Magnesium Carbonate Hydroxide Magnesium Carbonate And Hcl Includes kit list and safety instructions. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. The balanced chemical equation is: Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. Learn how magnesium. Magnesium Carbonate And Hcl.
From hadassah-blogmercer.blogspot.com
Ionic Equation of Magnesium and Hydrochloric Acid Magnesium Carbonate And Hcl Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between magnesium and. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. The balanced chemical equation is: Learn how to perform and analyse the experiment of magnesium. Magnesium Carbonate And Hcl.
From www.numerade.com
SOLVED A 6.53 g sample of a mixture of magnesium carbonate and Magnesium Carbonate And Hcl The web page explains the reactions, gives examples and shows diagrams and equations. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction. Magnesium Carbonate And Hcl.
From www.slideserve.com
PPT KS4 Energy Transfer in Reactions PowerPoint Presentation, free Magnesium Carbonate And Hcl The balanced chemical equation is: Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. Learn how magnesium carbonate (mgco3) reacts with hydrochloric acid (hcl) to produce carbon dioxide (co2), magnesium chloride (mgcl2) and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong. Magnesium Carbonate And Hcl.
From mavink.com
Magnesium Reacts With Hydrochloric Acid Magnesium Carbonate And Hcl Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. The web page explains the reactions, gives examples and shows diagrams and equations. Includes kit list and safety instructions. Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. When equal amounts of a strong acid. Magnesium Carbonate And Hcl.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Magnesium Carbonate And Hcl Learn how acids react with metals, bases, carbonates and metal hydroxides to produce salts. A class practical on reacting magnesium with hydrochloric acid and how to measure the rate of reaction. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. Mg_((s))+ 2hcl_((aq))rarrmgcl_(2(aq)) + h_(2(g) the reaction between. Magnesium Carbonate And Hcl.
From www.chegg.com
Solved A 17.2 g sample of a mixture of magnesium carbonate Magnesium Carbonate And Hcl The web page explains the reactions, gives examples and shows diagrams and equations. Learn how to perform and analyse the experiment of magnesium and dilute hydrochloric acid reacting to form magnesium chloride and. When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the. The balanced chemical equation is:. Magnesium Carbonate And Hcl.