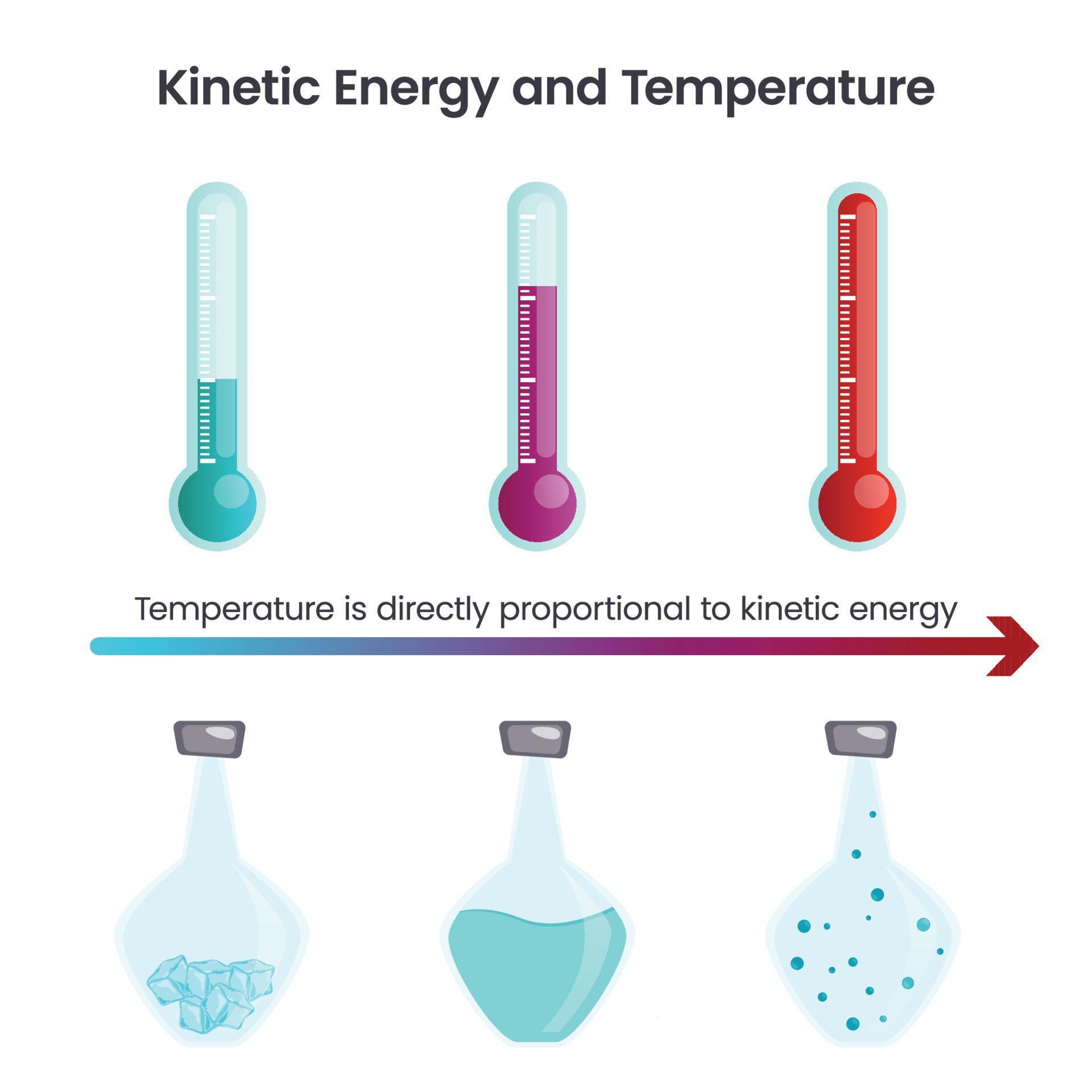
Metal Kinetic Energy Temperature . Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Define chemical energy and thermal energy. Define heat and work , and describe an important. Temperature is a quantitative measure of “hot”. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Thermal energy, temperature, and heat. Explain the difference between kinetic energy and potential energy. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased?
from www.vecteezy.com
Thermal energy, temperature, and heat. Define heat and work , and describe an important. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Define chemical energy and thermal energy. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Explain the difference between kinetic energy and potential energy. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Temperature is a quantitative measure of “hot”. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy.
Energy and Temperature science vector graphic 21790106 Vector
Metal Kinetic Energy Temperature Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Define heat and work , and describe an important. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Thermal energy, temperature, and heat. Temperature is a quantitative measure of “hot”. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Explain the difference between kinetic energy and potential energy. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Define chemical energy and thermal energy.
From www.slideserve.com
PPT Theory of Matter PowerPoint Presentation, free download Metal Kinetic Energy Temperature What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Thermal energy, temperature, and heat. Explain the difference between kinetic energy and potential energy. Define chemical energy and thermal energy. Define heat and work , and describe an important. Thermal energy is the kinetic energy associated with the random motion. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Lecture 3. The Molecular Theory of Gases PowerPoint Metal Kinetic Energy Temperature What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Temperature is a quantitative measure of “hot”. Define heat and work , and describe an important. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Thermal energy is the kinetic. Metal Kinetic Energy Temperature.
From ar.inspiredpencil.com
Energy Temperature Metal Kinetic Energy Temperature At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Thermal energy, temperature, and heat. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Explain the difference between kinetic energy and potential energy. Temperature is a quantitative measure. Metal Kinetic Energy Temperature.
From pdfslide.net
(PDF) Temperature and Energy Heat/Enthalpy …carussell.weebly Metal Kinetic Energy Temperature Explain the difference between kinetic energy and potential energy. Define chemical energy and thermal energy. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Define heat and work , and describe an important. Temperature is a quantitative measure of “hot”. Thermal energy is the kinetic energy associated. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Temperature measures the average energy of PowerPoint Metal Kinetic Energy Temperature At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Define heat and work , and describe an important. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. What is a kinetic energy distribution curve and how does the. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Thermal Energy A. Temperature & Heat 1. Temperature is related to Metal Kinetic Energy Temperature What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Define chemical energy and thermal energy. Define heat and work , and describe an important. Temperature is a quantitative measure of. Metal Kinetic Energy Temperature.
From slideplayer.com
Temperature Section ppt download Metal Kinetic Energy Temperature Temperature is a quantitative measure of “hot”. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Explain the difference between kinetic energy and potential energy. Thermal energy. Metal Kinetic Energy Temperature.
From www.atmo.arizona.edu
Lecture 11 Temperature, conduction, convection, and latent heat Metal Kinetic Energy Temperature Explain the difference between kinetic energy and potential energy. Temperature is a quantitative measure of “hot”. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Define chemical energy and thermal energy. Define. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Unit 6 PowerPoint Presentation, free download ID1516058 Metal Kinetic Energy Temperature Define chemical energy and thermal energy. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Explain the difference between kinetic energy and potential energy. Temperature is a quantitative measure of. Metal Kinetic Energy Temperature.
From www.youtube.com
Chapter 10 21 Energy is Related to Temperature YouTube Metal Kinetic Energy Temperature The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal. Metal Kinetic Energy Temperature.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Metal Kinetic Energy Temperature Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Explain the difference between kinetic energy and potential energy. Temperature is a quantitative measure of “hot”. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. What is a kinetic energy distribution curve and. Metal Kinetic Energy Temperature.
From www.tec-science.com
Why does the temperature remain constant during a change of state Metal Kinetic Energy Temperature Define heat and work , and describe an important. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Define chemical energy and thermal energy. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Temperature is not directly proportional to internal energy. Metal Kinetic Energy Temperature.
From www.vecteezy.com
Energy and Temperature science vector graphic 21790106 Vector Metal Kinetic Energy Temperature At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Temperature is a quantitative measure of “hot”. Define chemical energy and thermal energy. Define heat and work , and describe. Metal Kinetic Energy Temperature.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Metal Kinetic Energy Temperature Thermal energy, temperature, and heat. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Thermal energy is the kinetic energy associated with the random motion of atoms and. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Lecture 3. The Molecular Theory of Gases PowerPoint Metal Kinetic Energy Temperature What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Define heat and work , and describe an important. Thermal energy, temperature, and heat. Temperature is a quantitative measure of “hot”. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy.. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Energy PowerPoint Presentation, free download ID1551429 Metal Kinetic Energy Temperature Define chemical energy and thermal energy. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Thermal energy, temperature, and heat. Explain the difference between kinetic energy and potential energy. At any given temperature, not all of the particles of a sample of matter have the same kinetic. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint Metal Kinetic Energy Temperature What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Define chemical energy and thermal energy. Temperature is a quantitative measure of “hot”. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Explain the difference between kinetic energy and potential. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Chapter 10 PowerPoint Presentation, free download ID6805495 Metal Kinetic Energy Temperature What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Explain the difference between kinetic energy and potential energy. Thermal energy, temperature, and heat. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Temperature is not directly. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Chapter 21 Temperature, Heat and Expansion PowerPoint Metal Kinetic Energy Temperature Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Define heat and work , and describe an important. What is a kinetic energy distribution curve and how does the shape of the. Metal Kinetic Energy Temperature.
From www.youtube.com
Energy, Temperature and Particle Speeds YouTube Metal Kinetic Energy Temperature Thermal energy, temperature, and heat. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Define heat and work , and describe an important. Temperature is a quantitative measure of “hot”. The kinetic temperature is. Metal Kinetic Energy Temperature.
From ar.inspiredpencil.com
Energy Diagram Temperature Metal Kinetic Energy Temperature Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Define heat and work , and describe an important. Define chemical energy and thermal energy. Temperature is a quantitative measure of “hot”. Explain the. Metal Kinetic Energy Temperature.
From www.studypool.com
SOLUTION Temperature energy Studypool Metal Kinetic Energy Temperature Define heat and work , and describe an important. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Thermal energy, temperature, and heat. Temperature is a quantitative measure of “hot”. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? The kinetic temperature is. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Energy, Temperature, Phase Changes PowerPoint Metal Kinetic Energy Temperature Define chemical energy and thermal energy. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Temperature is a quantitative measure of “hot”. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Define heat and work , and describe an important. Explain the. Metal Kinetic Energy Temperature.
From www.studypool.com
SOLUTION Temperature energy Studypool Metal Kinetic Energy Temperature Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Explain the difference between kinetic energy and potential energy. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. What is a kinetic energy distribution curve and how does the shape of the curve. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Chapter 7 Energy and Chemical Change PowerPoint Presentation Metal Kinetic Energy Temperature At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Temperature is a quantitative measure of “hot”. Define heat and work , and describe an important. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Thermal energy is the kinetic. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Matter and Temperature PowerPoint Presentation, free download Metal Kinetic Energy Temperature Explain the difference between kinetic energy and potential energy. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Thermal energy, temperature, and heat. The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Temperature is a quantitative measure of “hot”. Define chemical. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Temperature measures the average energy of PowerPoint Metal Kinetic Energy Temperature Explain the difference between kinetic energy and potential energy. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Thermal energy, temperature, and heat. Define heat and work , and describe an important. Define chemical energy and thermal energy. Temperature is a quantitative measure of “hot”. Thermal energy is. Metal Kinetic Energy Temperature.
From www.researchgate.net
Temperature versus energy. Temperature and the quasistatic Metal Kinetic Energy Temperature Thermal energy, temperature, and heat. Temperature is a quantitative measure of “hot”. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Define chemical energy and thermal energy. Explain. Metal Kinetic Energy Temperature.
From www.youtube.com
6.1 Temperature and energy (SL) YouTube Metal Kinetic Energy Temperature Temperature is a quantitative measure of “hot”. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Explain the difference between kinetic energy and potential energy. Define heat and work , and describe an important. What is a kinetic energy distribution curve and how does the shape of the curve change. Metal Kinetic Energy Temperature.
From www.youtube.com
Temperature & Energy YouTube Metal Kinetic Energy Temperature Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Thermal energy, temperature, and heat. Define heat and work , and describe an important. Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. The kinetic temperature is the variable needed for subjects like. Metal Kinetic Energy Temperature.
From www.inspiritvr.com
Average Energy and Temperature Study Guide Inspirit Metal Kinetic Energy Temperature Explain the difference between kinetic energy and potential energy. Define chemical energy and thermal energy. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. Temperature is not. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT Temperature v. Heat PowerPoint Presentation ID1701293 Metal Kinetic Energy Temperature Define heat and work , and describe an important. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Temperature is not directly proportional to internal energy since temperature measures only the kinetic energy part of the internal energy, so. Thermal energy, temperature, and heat. Explain the difference between kinetic. Metal Kinetic Energy Temperature.
From www.slideserve.com
PPT THEORY PowerPoint Presentation, free download ID6790727 Metal Kinetic Energy Temperature Temperature is a quantitative measure of “hot”. Thermal energy is the kinetic energy associated with the random motion of atoms and molecules. Thermal energy, temperature, and heat. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. What is a kinetic energy distribution curve and how does the shape of the. Metal Kinetic Energy Temperature.
From haipernews.com
How To Calculate Energy Thermal Haiper Metal Kinetic Energy Temperature The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. What is a kinetic energy distribution curve and how does the shape of the curve change when temperature is increased? Explain the difference between kinetic energy and potential energy. Temperature is a quantitative measure of “hot”. Define chemical. Metal Kinetic Energy Temperature.
From energyeducation.ca
Thermal conduction Energy Education Metal Kinetic Energy Temperature The kinetic temperature is the variable needed for subjects like heat transfer, because it is the translational kinetic energy which leads to. At any given temperature, not all of the particles of a sample of matter have the same kinetic energy. Thermal energy, temperature, and heat. Temperature is not directly proportional to internal energy since temperature measures only the kinetic. Metal Kinetic Energy Temperature.