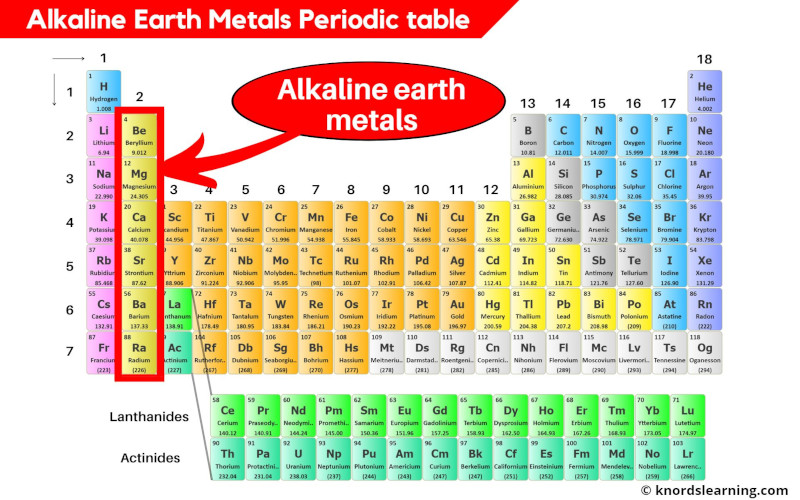
How To Identify Alkaline Earth Metals . Are the alkaline earth elements more or less reactive than the alkali. Alkaline earths have low electron affinities and low electronegativities. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. How many electrons are in the outer shell of the alkaline earth elements? As with the alkali metals, the properties depend on the ease with which electrons are lost.
from knordslearning.com
The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. How many electrons are in the outer shell of the alkaline earth elements? As with the alkali metals, the properties depend on the ease with which electrons are lost. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earths have low electron affinities and low electronegativities. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Are the alkaline earth elements more or less reactive than the alkali.
Alkaline Earth Metals Periodic Table (With Images)
How To Identify Alkaline Earth Metals Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earths have low electron affinities and low electronegativities. The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. How many electrons are in the outer shell of the alkaline earth elements? Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Are the alkaline earth elements more or less reactive than the alkali. As with the alkali metals, the properties depend on the ease with which electrons are lost.
From www.slideserve.com
PPT Elements and their Properties PowerPoint Presentation, free download ID6909242 How To Identify Alkaline Earth Metals As with the alkali metals, the properties depend on the ease with which electrons are lost. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth. How To Identify Alkaline Earth Metals.
From cabinet.matttroy.net
Alkaline Earth Metals Periodic Table Definition Matttroy How To Identify Alkaline Earth Metals Are the alkaline earth elements more or less reactive than the alkali. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. How many electrons are in the outer shell of the alkaline earth elements? Alkaline earths have low electron affinities and low electronegativities. The alkaline. How To Identify Alkaline Earth Metals.
From www.preciseceramic.com
Six Alkaline Earth Metals & Their Oxides How To Identify Alkaline Earth Metals How many electrons are in the outer shell of the alkaline earth elements? The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2].. How To Identify Alkaline Earth Metals.
From www.britannica.com
Alkalineearth metal chemical element Britannica How To Identify Alkaline Earth Metals The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Alkaline earths have low electron affinities and low electronegativities. How many electrons are. How To Identify Alkaline Earth Metals.
From www.animalia-life.club
Periodic Table Of Elements Alkaline Earth Metals How To Identify Alkaline Earth Metals How many electrons are in the outer shell of the alkaline earth elements? Alkaline earths have low electron affinities and low electronegativities. Are the alkaline earth elements more or less reactive than the alkali. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Although all the alkaline metals are found in. How To Identify Alkaline Earth Metals.
From elchoroukhost.net
Alkali Metals Periodic Table Location Elcho Table How To Identify Alkaline Earth Metals As with the alkali metals, the properties depend on the ease with which electrons are lost. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. How many electrons are in the outer shell of the alkaline earth elements? The alkaline earth metals or alkaline earths are a set of six elements. How To Identify Alkaline Earth Metals.
From www.slideserve.com
PPT Alkali Metals and Alkaline Earth Metals PowerPoint Presentation, free download ID2873159 How To Identify Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. As with the alkali metals, the properties depend on the ease with which electrons are lost. Although all the alkaline metals are found in. How To Identify Alkaline Earth Metals.
From www.slideserve.com
PPT The Periodic Table PowerPoint Presentation, free download ID2352439 How To Identify Alkaline Earth Metals As with the alkali metals, the properties depend on the ease with which electrons are lost. Are the alkaline earth elements more or less reactive than the alkali. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are a group of highly reactive elements placed right next to. How To Identify Alkaline Earth Metals.
From www.vedantu.com
Alkali Metals Chemical Elements, Properties Alkali Metals Periodic Table How To Identify Alkaline Earth Metals How many electrons are in the outer shell of the alkaline earth elements? Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earths have low electron affinities and low electronegativities. Are the alkaline earth elements more or less reactive than the alkali. As with the alkali metals, the properties depend. How To Identify Alkaline Earth Metals.
From www.slideserve.com
PPT Ch 5 Atomic Structure and the Periodic Table PowerPoint Presentation ID6165304 How To Identify Alkaline Earth Metals As with the alkali metals, the properties depend on the ease with which electrons are lost. Alkaline earths have low electron affinities and low electronegativities. Are the alkaline earth elements more or less reactive than the alkali. The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table.. How To Identify Alkaline Earth Metals.
From elchoroukhost.net
Alkali Metals Periodic Table Properties Elcho Table How To Identify Alkaline Earth Metals Alkaline earths have low electron affinities and low electronegativities. Are the alkaline earth elements more or less reactive than the alkali. How many electrons are in the outer shell of the alkaline earth elements? Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals or alkaline earths are. How To Identify Alkaline Earth Metals.
From xlskoor.blogspot.com
Alkali Metals Chemistry How To Identify Alkaline Earth Metals The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two. How To Identify Alkaline Earth Metals.
From slidetodoc.com
Alkali Metals and the Alkaline Earth metals www How To Identify Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Are the alkaline earth elements more or less reactive than the alkali. The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. How many electrons are in the outer. How To Identify Alkaline Earth Metals.
From www.sliderbase.com
Element Classes Presentation Chemistry How To Identify Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Are. How To Identify Alkaline Earth Metals.
From www.nagwa.com
Question Video Identifying Alkaline Earth Metals Nagwa How To Identify Alkaline Earth Metals Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. As with the alkali metals, the properties depend on the ease with which electrons are lost. Are the alkaline earth elements more or less. How To Identify Alkaline Earth Metals.
From www.youtube.com
Occurrence of ALKALI & ALKALINE Earth Metals SBlock Elements F.Sc 2nd Year Chemistry How To Identify Alkaline Earth Metals Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. How many electrons are in the outer shell of the alkaline earth elements? Alkaline earths have low electron affinities and low electronegativities. The alkaline. How To Identify Alkaline Earth Metals.
From cabinet.matttroy.net
Alkaline Earth Metals Periodic Table Definition Matttroy How To Identify Alkaline Earth Metals Alkaline earths have low electron affinities and low electronegativities. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. As with the alkali metals, the properties depend on. How To Identify Alkaline Earth Metals.
From scienceinfo.com
Comparison of properties of Alkali and Alkaline Earth Metals How To Identify Alkaline Earth Metals As with the alkali metals, the properties depend on the ease with which electrons are lost. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earths have low electron affinities and low electronegativities. Are the alkaline earth elements more or less reactive than the alkali. Although all the alkaline metals. How To Identify Alkaline Earth Metals.
From examples.yourdictionary.com
Basic Types of Metals on the Periodic Table How To Identify Alkaline Earth Metals As with the alkali metals, the properties depend on the ease with which electrons are lost. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Are the alkaline earth elements more or less. How To Identify Alkaline Earth Metals.
From byjus.com
Alkaline Earth Metals Occurrence and Extraction,Physical Properties How To Identify Alkaline Earth Metals Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Are the alkaline earth elements more or less reactive than the alkali. As with the alkali metals, the properties depend on the ease with which electrons are lost. The alkaline earth metals or alkaline earths are. How To Identify Alkaline Earth Metals.
From www.expii.com
Alkaline Earth Metals — Overview & Properties Expii How To Identify Alkaline Earth Metals The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. As with the alkali metals, the properties depend on the ease with which electrons are lost. Although all the. How To Identify Alkaline Earth Metals.
From www.askiitians.com
Alkaline Earth Metals Study Material for IIT JEE askIITians How To Identify Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. As with the alkali metals, the properties depend on the ease with which electrons are lost. Although all the alkaline metals are found in. How To Identify Alkaline Earth Metals.
From pediaa.com
Difference Between Alkali Metals and Alkaline Earth Metals Definition, Properties, Examples How To Identify Alkaline Earth Metals Are the alkaline earth elements more or less reactive than the alkali. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. As with the alkali metals, the properties depend on the ease with which electrons are lost. Alkaline earths have low electron affinities and low electronegativities. How many electrons are in. How To Identify Alkaline Earth Metals.
From knordslearning.com
Alkaline Earth Metals Periodic Table (With Images) How To Identify Alkaline Earth Metals Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earths have low electron affinities and low electronegativities. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. How many electrons are in the outer shell of the alkaline earth elements? As with. How To Identify Alkaline Earth Metals.
From utedzz.blogspot.com
Periodic Table Showing Alkali Metals Alkaline Earth Metals Periodic Table Timeline How To Identify Alkaline Earth Metals Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Are the alkaline earth elements more or less reactive than the alkali. The alkaline earth metals or alkaline. How To Identify Alkaline Earth Metals.
From study.com
How to Identify Alkali Metals, Alkaline Earth Metals & Transition Metals using the Periodic How To Identify Alkaline Earth Metals Are the alkaline earth elements more or less reactive than the alkali. Alkaline earths have low electron affinities and low electronegativities. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure. How To Identify Alkaline Earth Metals.
From byjus.com
To which group do the alkaline earth metals belong? How To Identify Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Alkaline earths have low electron affinities and low electronegativities. Alkaline earth metals are a group of highly reactive elements. How To Identify Alkaline Earth Metals.
From www.tes.com
Alkali and Alkaline Earth Metals Venn Diagram Teaching Resources How To Identify Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. How many electrons are in the outer shell of the alkaline earth elements? Are the alkaline earth elements more or less reactive than the alkali. The alkaline earth metals or alkaline earths are a set of six elements found in the second. How To Identify Alkaline Earth Metals.
From www.youtube.com
Properties of the Alkaline Earth Metals YouTube How To Identify Alkaline Earth Metals Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. How many electrons are in the outer shell of the alkaline earth elements? Are the alkaline earth elements more or less reactive than the alkali. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal. How To Identify Alkaline Earth Metals.
From www.breakingatom.com
Group 2 The Alkaline Earth Metals How To Identify Alkaline Earth Metals As with the alkali metals, the properties depend on the ease with which electrons are lost. Are the alkaline earth elements more or less reactive than the alkali. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. How many electrons are in the outer shell of the alkaline earth elements? Although. How To Identify Alkaline Earth Metals.
From byjus.com
Are Alkaline Earth Metals Soft or Hard? How To Identify Alkaline Earth Metals Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. Alkaline earths have low electron affinities and low electronegativities. Alkaline earth metals dissolve in liquid ammonia to give solutions that contain two solvated electrons per metal atom. Are the alkaline earth elements more or less reactive than the alkali. How many electrons. How To Identify Alkaline Earth Metals.
From saadworldwide.weebly.com
Periodic table Alkaline earth metals definition chemistry saadworldwide How To Identify Alkaline Earth Metals How many electrons are in the outer shell of the alkaline earth elements? The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Are the alkaline earth elements more or less reactive than the alkali. Alkaline earth metals are a group of highly reactive elements placed right. How To Identify Alkaline Earth Metals.
From newtondesk.com
Alkaline Earth Metals On The Periodic Table Chemistry Elements How To Identify Alkaline Earth Metals Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. The alkaline earth metals or alkaline earths are a set of six elements found in the second group (column) of the periodic table. Are the alkaline earth elements more or less reactive than the alkali. Alkaline. How To Identify Alkaline Earth Metals.
From www.animalia-life.club
Periodic Table Of Elements Alkaline Earth Metals How To Identify Alkaline Earth Metals How many electrons are in the outer shell of the alkaline earth elements? Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. As with the alkali metals, the properties depend on the ease with which electrons are lost. Alkaline earth metals dissolve in liquid ammonia. How To Identify Alkaline Earth Metals.
From www.youtube.com
ALKALINE EARTH METALS YouTube How To Identify Alkaline Earth Metals Although all the alkaline metals are found in nature, their high reactivity prevents them from occuring freely or in their pure form [1, 2]. Alkaline earth metals are a group of highly reactive elements placed right next to the alkali metal group. As with the alkali metals, the properties depend on the ease with which electrons are lost. Are the. How To Identify Alkaline Earth Metals.