Use Standard Enthalpies Of Formation From Table R11 To Calculate . explore the foundations of thermochemistry with our standard formation enthalpy calculator! 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2.
from www.chegg.com
— use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. explore the foundations of thermochemistry with our standard formation enthalpy calculator! by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its.
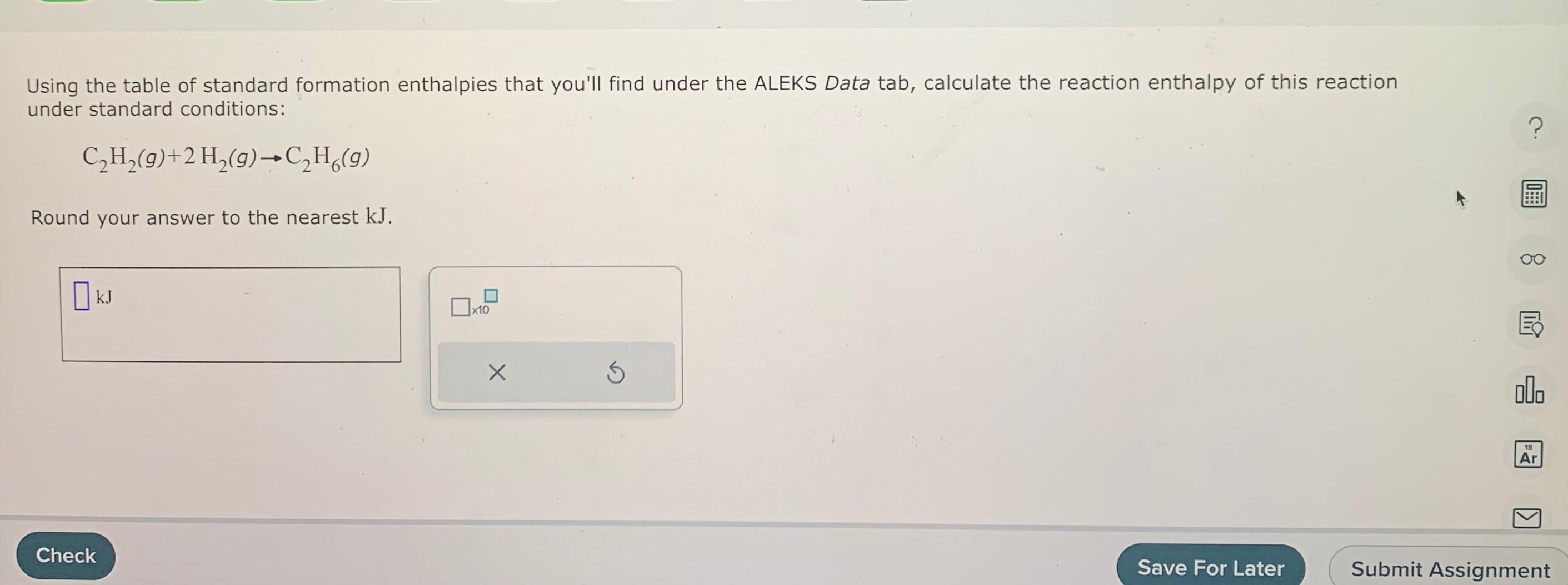
Solved Using the table of standard formation enthalpies that
Use Standard Enthalpies Of Formation From Table R11 To Calculate by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. explore the foundations of thermochemistry with our standard formation enthalpy calculator! — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\).
From www.chegg.com
Solved Use standard enthalpies of formation from the Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. explore the foundations of thermochemistry with our standard formation enthalpy calculator! 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of.. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From schoolworkhelper.net
Standard Enthalpies of Formation Online Homework Help SchoolWorkHelper Use Standard Enthalpies Of Formation From Table R11 To Calculate — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. using the values in the above table of standard enthalpies of formation, calculate the δh reaction. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.solutionspile.com
[Solved] Using the standard enthalpies of formation liste Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. — use the data in table t1 to calculate the standard. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using The Table Of Standard Formation Enthalpies T... Use Standard Enthalpies Of Formation From Table R11 To Calculate explore the foundations of thermochemistry with our standard formation enthalpy calculator! using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. —. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.numerade.com
SOLVED Using Standard Enthalpy of Formation Enthalpy Test (all Use Standard Enthalpies Of Formation From Table R11 To Calculate explore the foundations of thermochemistry with our standard formation enthalpy calculator! by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). — use the data in table t1 to calculate the. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). using the values in the above table of standard enthalpies of formation, calculate the δh reaction o. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.solutionspile.com
[Solved] Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. explore the foundations of thermochemistry with our standard formation enthalpy calculator!. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). explore the foundations of thermochemistry with our standard formation enthalpy calculator! the standard enthalpy of formation, also known as the heat of. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. by definition, the standard enthalpy of formation of an element in. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From exoyndeil.blob.core.windows.net
Standard Enthalpy Of Formation In Elements at Michael Zapien blog Use Standard Enthalpies Of Formation From Table R11 To Calculate — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the standard enthalpy of formation, also known as the heat of formation, is the. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.numerade.com
SOLVED Calculating a molar heat of reaction from formation enthalpies Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. explore the foundations of thermochemistry with our standard formation enthalpy calculator! by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. — standard enthalpies of formation for some common compounds are. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Table 1. Standard enthalpies of formation for Use Standard Enthalpies Of Formation From Table R11 To Calculate using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of.. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.numerade.com
SOLVED Using the table of standard formation enthalpies that you"Il Use Standard Enthalpies Of Formation From Table R11 To Calculate 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. using the values in the above table of standard enthalpies of. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.numerade.com
SOLVED Calculate values for the standard reaction enthalpies at 298k Use Standard Enthalpies Of Formation From Table R11 To Calculate explore the foundations of thermochemistry with our standard formation enthalpy calculator! — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. — use the data in table t1 to calculate the. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Use Standard Enthalpies Of Formation From Table R11 To Calculate explore the foundations of thermochemistry with our standard formation enthalpy calculator! — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. — standard enthalpies of. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. — standard enthalpies of formation for some common compounds are given. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. explore the foundations of thermochemistry with our standard formation enthalpy calculator! the standard. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. explore the foundations of thermochemistry with our standard formation enthalpy. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Use Standard Enthalpies Of Formation From Table R11 To Calculate explore the foundations of thermochemistry with our standard formation enthalpy calculator! — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. — standard enthalpies of formation. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.numerade.com
SOLVEDUse standard enthalpies of formation from Table 7.2 to determine Use Standard Enthalpies Of Formation From Table R11 To Calculate — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. explore the foundations of thermochemistry with our standard formation enthalpy calculator! by definition, the standard enthalpy. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate explore the foundations of thermochemistry with our standard formation enthalpy calculator! — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. by definition, the standard enthalpy of formation of. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Use a standard enthalpies of formation table to Use Standard Enthalpies Of Formation From Table R11 To Calculate using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. 193 rows — the standard enthalpy change of. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. explore the foundations of thermochemistry with our standard formation enthalpy calculator! — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). — use the data in. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Use enthalpies of formation to calculate the standard Use Standard Enthalpies Of Formation From Table R11 To Calculate — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. using the values in the above table of standard enthalpies of. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. explore the foundations of thermochemistry with our standard formation enthalpy calculator! — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). by definition, the standard enthalpy. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard enthalpies of formation Use Standard Enthalpies Of Formation From Table R11 To Calculate — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Use Standard Enthalpies Of Formation From Table R11 To Calculate explore the foundations of thermochemistry with our standard formation enthalpy calculator! using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). — use the data in table t1 to. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using The Table Of Standard Formation Enthalpies T... Use Standard Enthalpies Of Formation From Table R11 To Calculate by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). explore. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.numerade.com
Using the table of standard formation enthalpies that you'll find under Use Standard Enthalpies Of Formation From Table R11 To Calculate 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. by definition,. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation From Table R11 To Calculate — use the data in table t1 to calculate the standard enthalpy of formation of ammonium sulfate (in kilojoules per. explore the foundations of thermochemistry with our standard formation enthalpy calculator! 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. the standard enthalpy of formation,. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From kunduz.com
[ANSWERED] Use the standard enthalpies of formation to calculate the AH Use Standard Enthalpies Of Formation From Table R11 To Calculate — standard enthalpies of formation for some common compounds are given in table \(\pageindex{1}\). explore the foundations of thermochemistry with our standard formation enthalpy calculator! 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. — use the data in table t1 to calculate the standard. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Use Standard Enthalpies Of Formation From Table R11 To Calculate by definition, the standard enthalpy of formation of an element in its most stable form (most stable allotrope) is equal to. 193 rows — the standard enthalpy change of any reaction can be calculated from the standard enthalpies of formation of. explore the foundations of thermochemistry with our standard formation enthalpy calculator! using the values in. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. explore the foundations of thermochemistry with our standard formation enthalpy calculator! the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance. Use Standard Enthalpies Of Formation From Table R11 To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation From Table R11 To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. — standard enthalpies of formation for some common. Use Standard Enthalpies Of Formation From Table R11 To Calculate.