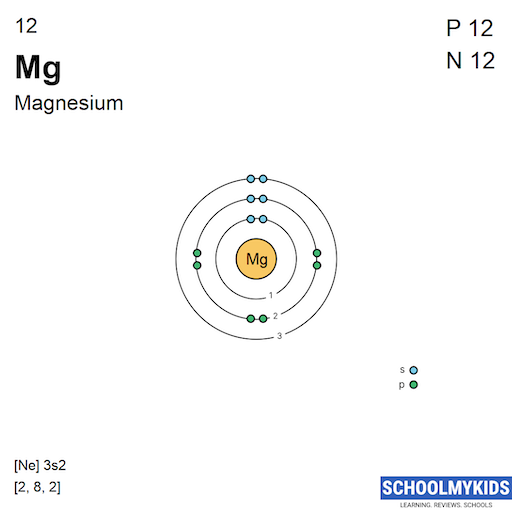
Magnesium Energy Levels Electron . Magnesium has two valence electrons in the 3s orbital. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. How are ionisation energies linked to the main electron energy levels ? 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). The first two electrons are found in the n = 1 n = 1 energy level, the next eight. A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; Notice the big jump between 4 and 5. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. To form a magnesium ion, it loses its two. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The ground state electron configuration of magnesium is important in understanding its. Magnesium has 12 electrons arranged in three energy levels.
from www.schoolmykids.com
The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². How are ionisation energies linked to the main electron energy levels ? When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. Notice the big jump between 4 and 5. Magnesium has two valence electrons in the 3s orbital. 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). To form a magnesium ion, it loses its two. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Magnesium has 12 electrons arranged in three energy levels.
Magnesium (Mg) Element Information, Facts, Properties, Uses
Magnesium Energy Levels Electron A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; Notice the big jump between 4 and 5. The ground state electron configuration of magnesium is important in understanding its. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Magnesium has 12 electrons arranged in three energy levels. Magnesium has two valence electrons in the 3s orbital. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. To form a magnesium ion, it loses its two. A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). How are ionisation energies linked to the main electron energy levels ?
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Magnesium Energy Levels Electron 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The. Magnesium Energy Levels Electron.
From www.istockphoto.com
Mg Magnesium Element Information Facts Properties Trends Uses And Magnesium Energy Levels Electron When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The first two electrons are found in the n = 1 n =. Magnesium Energy Levels Electron.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents Magnesium Energy Levels Electron How are ionisation energies linked to the main electron energy levels ? Magnesium has 12 electrons arranged in three energy levels. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. To form a magnesium ion, it loses its two. Magnesium has two valence electrons in the 3s orbital. Let's look. Magnesium Energy Levels Electron.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Magnesium Energy Levels Electron The first two electrons are found in the n = 1 n = 1 energy level, the next eight. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; 19 rows research has shown that unpaired electrons (a single electron. Magnesium Energy Levels Electron.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Energy Levels Electron Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. To form a magnesium ion, it loses its two. 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). Magnesium has 12 electrons arranged. Magnesium Energy Levels Electron.
From www.mikrora.com
File Electron Configuration Magnesium Svg Best Diagram Collection Magnesium Energy Levels Electron How are ionisation energies linked to the main electron energy levels ? The first two electrons are found in the n = 1 n = 1 energy level, the next eight. 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital).. Magnesium Energy Levels Electron.
From www.youtube.com
Electronic configuration for Magnesium (Mg) spdf Trick Chemistry Magnesium Energy Levels Electron When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; Magnesium has 12 electrons arranged in three energy levels. To. Magnesium Energy Levels Electron.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Energy Levels Electron Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Notice the big jump between 4 and 5. 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). The ground state electron configuration of. Magnesium Energy Levels Electron.
From www.researchgate.net
Energylevel diagram for magnesium. The full length horizontal lines Magnesium Energy Levels Electron Magnesium has 12 electrons arranged in three energy levels. 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; How are. Magnesium Energy Levels Electron.
From brainly.com
The element magnesium has 12 electrons. In how many energy levels are Magnesium Energy Levels Electron A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; The first two electrons are found in the n = 1 n = 1 energy level, the next eight. To form a magnesium ion, it loses its two. When writing an electron configuration, first write the energy level (the period),. Magnesium Energy Levels Electron.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Magnesium Energy Levels Electron Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The first two electrons are found in the n = 1 n = 1 energy level, the next eight. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Magnesium has 12 electrons arranged. Magnesium Energy Levels Electron.
From www.dreamstime.com
Magnesium Atom, with Mass and Energy Levels. Stock Vector Magnesium Energy Levels Electron How are ionisation energies linked to the main electron energy levels ? A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. Notice the big jump between 4 and 5. The electron configuration. Magnesium Energy Levels Electron.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Magnesium Energy Levels Electron Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). When writing an electron configuration, first write the energy level (the period), then the. Magnesium Energy Levels Electron.
From www.pinterest.co.uk
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Energy Levels Electron The ground state electron configuration of magnesium is important in understanding its. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. How are. Magnesium Energy Levels Electron.
From brainly.ph
How many core electrons of Magnesium 12 (12Mg) Brainly.ph Magnesium Energy Levels Electron Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The ground state electron configuration of magnesium is important in understanding. Magnesium Energy Levels Electron.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Magnesium Energy Levels Electron 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). Magnesium has 12 electrons arranged in three energy levels. To form a magnesium ion, it loses its two. The ground state electron configuration of magnesium is important in understanding its. Magnesium. Magnesium Energy Levels Electron.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Energy Levels Electron A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is. Magnesium Energy Levels Electron.
From mungfali.com
Magnesium Orbital Diagram Magnesium Energy Levels Electron The ground state electron configuration of magnesium is important in understanding its. How are ionisation energies linked to the main electron energy levels ? To form a magnesium ion, it loses its two. Notice the big jump between 4 and 5. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Magnesium Energy Levels Electron.
From joizkfdrp.blob.core.windows.net
Magnesium Ion Aufbau Diagram at Katherine Cortez blog Magnesium Energy Levels Electron A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Notice the big jump between 4 and 5. How are ionisation energies linked to the main electron energy levels ? To form a magnesium ion, it loses its two. When. Magnesium Energy Levels Electron.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Magnesium Energy Levels Electron The ground state electron configuration of magnesium is important in understanding its. Magnesium has two valence electrons in the 3s orbital. To form a magnesium ion, it loses its two. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The first two electrons are found in the n = 1 n = 1 energy level, the next eight. 19. Magnesium Energy Levels Electron.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Magnesium Energy Levels Electron 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). Magnesium has two valence electrons in the 3s orbital. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. How are ionisation. Magnesium Energy Levels Electron.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Magnesium Energy Levels Electron When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. Let's look at the figure below which shows the electron diagram. Magnesium Energy Levels Electron.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Magnesium Energy Levels Electron The ground state electron configuration of magnesium is important in understanding its. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The first two electrons are found in the n = 1 n = 1 energy level, the next eight. Magnesium has 12 electrons arranged in three energy levels. When writing an electron configuration, first write the energy level. Magnesium Energy Levels Electron.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Magnesium Energy Levels Electron The ground state electron configuration of magnesium is important in understanding its. Notice the big jump between 4 and 5. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; The first two electrons are found in the n =. Magnesium Energy Levels Electron.
From www.istockphoto.com
Mg Magnesium Element Information Facts Properties Trends Uses And Magnesium Energy Levels Electron Magnesium has 12 electrons arranged in three energy levels. The ground state electron configuration of magnesium is important in understanding its. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The electron configuration of magnesium is 1s² 2s² 2p⁶. Magnesium Energy Levels Electron.
From www.researchgate.net
Energylevel diagram for magnesium showing the levels relevant for this Magnesium Energy Levels Electron Magnesium has 12 electrons arranged in three energy levels. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. How are ionisation energies linked to the main electron energy levels ? To form a magnesium ion, it loses its two. 19 rows research has shown that unpaired electrons (a single electron. Magnesium Energy Levels Electron.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Magnesium Energy Levels Electron When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. 19 rows research has shown that unpaired electrons (a single electron. Magnesium Energy Levels Electron.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Magnesium Energy Levels Electron The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. Notice the big jump between 4 and 5. Magnesium has 12 electrons arranged in three energy levels. The first two. Magnesium Energy Levels Electron.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Magnesium Energy Levels Electron The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². How are ionisation energies linked to the main electron energy levels ? To form a magnesium ion, it loses its two. The ground state electron configuration of magnesium is important in understanding its. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons.. Magnesium Energy Levels Electron.
From mungfali.com
Magnesium Orbital Diagram Magnesium Energy Levels Electron A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². When writing an electron configuration, first write the energy level (the period), then. Magnesium Energy Levels Electron.
From www.dreamstime.com
Model of magnesium atom stock vector. Illustration of mass 164475021 Magnesium Energy Levels Electron The first two electrons are found in the n = 1 n = 1 energy level, the next eight. How are ionisation energies linked to the main electron energy levels ? Magnesium has 12 electrons arranged in three energy levels. A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2;. Magnesium Energy Levels Electron.
From www.alamy.com
Magnesium atom, with mass and energy levels. Vector illustration Stock Magnesium Energy Levels Electron When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The ground state electron configuration of magnesium is important in understanding its. To form a magnesium ion, it loses its two. A magnesium atom has 12 electrons so its electronic. Magnesium Energy Levels Electron.
From www.vectorstock.com
Symbol and electron diagram for magnesium Vector Image Magnesium Energy Levels Electron Magnesium has 12 electrons arranged in three energy levels. A magnesium atom has 12 electrons so its electronic configuration would be 1s 2 2s 2 2p 6 3s 2; The ground state electron configuration of magnesium is important in understanding its. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has two valence electrons in the 3s orbital.. Magnesium Energy Levels Electron.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Magnesium Energy Levels Electron Notice the big jump between 4 and 5. The first two electrons are found in the n = 1 n = 1 energy level, the next eight. Magnesium has two valence electrons in the 3s orbital. Let's look at the figure below which shows the electron diagram for magnesium and its 12 electrons. The electron configuration of magnesium is 1s². Magnesium Energy Levels Electron.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Energy Levels Electron 19 rows research has shown that unpaired electrons (a single electron in an orbital) are in a lower energy configuration than are paired electrons (two electrons in an orbital). Magnesium has two valence electrons in the 3s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². To form a magnesium ion, it loses its two. The first two. Magnesium Energy Levels Electron.