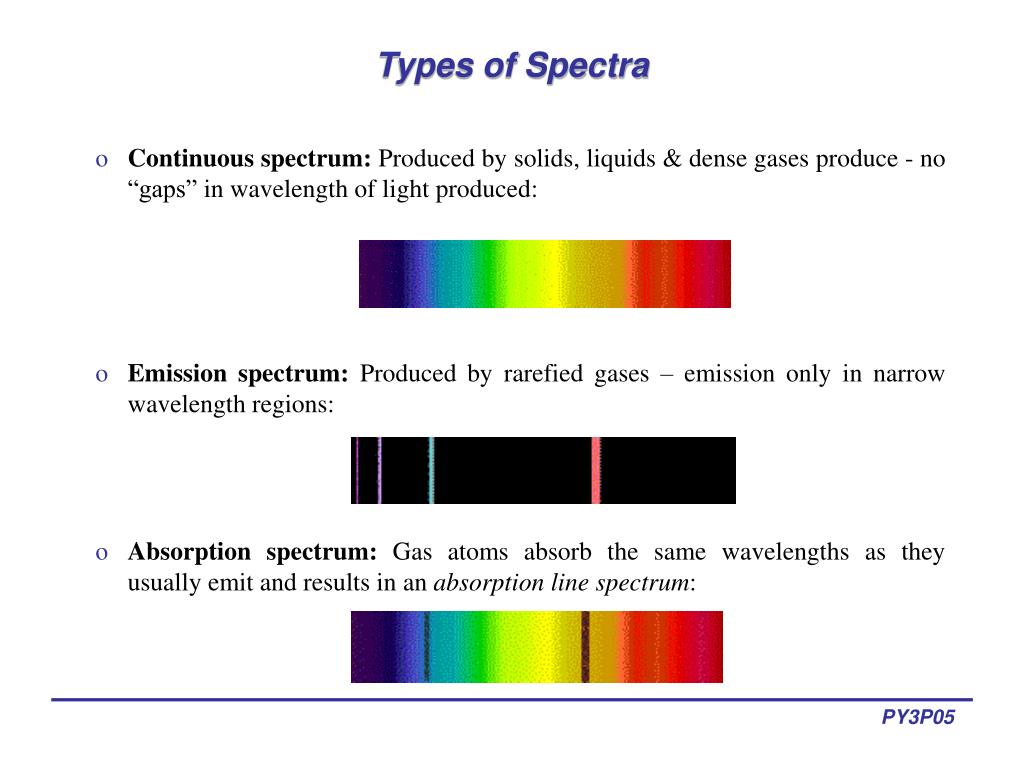
What Is Line And Band Spectra . A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. The shapes of these emission spectra fall into two broad types: These discrete lines arise due to the. Band spectra, or molecular spectra, are produced by molecules radiating. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Band spectrum refers to continuous spectrum with bands, typical of molecules, due.
from www.slideserve.com
In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. These discrete lines arise due to the. Band spectra, or molecular spectra, are produced by molecules radiating. The shapes of these emission spectra fall into two broad types: Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines.
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint
What Is Line And Band Spectra Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. These discrete lines arise due to the. The shapes of these emission spectra fall into two broad types: Band spectrum refers to continuous spectrum with bands, typical of molecules, due. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Band spectra, or molecular spectra, are produced by molecules radiating. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum What Is Line And Band Spectra Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. These discrete. What Is Line And Band Spectra.
From www.slideserve.com
PPT Honors Chemistry Unit 3 Electrons PowerPoint Presentation, free What Is Line And Band Spectra A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. What Is Line And Band Spectra.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning What Is Line And Band Spectra Band spectrum refers to continuous spectrum with bands, typical of molecules, due. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. The shapes of these emission spectra fall into two broad types: Band spectra, or molecular spectra, are produced by molecules radiating. The result is an emission spectrum that shows the intensity. What Is Line And Band Spectra.
From ar.inspiredpencil.com
Incandescent Light Emission Spectrum What Is Line And Band Spectra Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line. What Is Line And Band Spectra.
From www.researchgate.net
Kband spectra of the different classes of objects found in our What Is Line And Band Spectra These discrete lines arise due to the. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Band spectra, or molecular spectra, are produced by molecules radiating. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a. What Is Line And Band Spectra.
From www.researchgate.net
The emission spectrum of N2(CB) band ∆ν = −2 and ∆ν = −3 marked by What Is Line And Band Spectra These discrete lines arise due to the. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. The shapes of these emission. What Is Line And Band Spectra.
From www.youtube.com
2.3.2 Distinguish between a continuous spectrum and a line spectrum What Is Line And Band Spectra A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. The result. What Is Line And Band Spectra.
From pediaa.com
Difference Between Continuous Spectrum and Line Spectrum What Is Line And Band Spectra Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Although objects. What Is Line And Band Spectra.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom What Is Line And Band Spectra A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. In physics,. What Is Line And Band Spectra.
From www.nagwa.com
Question Video Identifying a Spectrum as an Emission or Absorption What Is Line And Band Spectra These discrete lines arise due to the. The shapes of these emission spectra fall into two broad types: Band spectra, or molecular spectra, are produced by molecules radiating. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. In physics, a ‘line spectrum’ is a set of discrete. What Is Line And Band Spectra.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint What Is Line And Band Spectra A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous. What Is Line And Band Spectra.
From imagine.gsfc.nasa.gov
Spectra Introduction What Is Line And Band Spectra In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. The result is an emission spectrum that shows the intensity of emission as a. What Is Line And Band Spectra.
From sdsu-physics.org
Quantum Physics What Is Line And Band Spectra These discrete lines arise due to the. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. Band spectra is the name given to. What Is Line And Band Spectra.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum What Is Line And Band Spectra The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. The shapes of these emission spectra fall into two broad types: Line spectrum refers to discrete set of. What Is Line And Band Spectra.
From www.slideserve.com
PPT What is Light? PowerPoint Presentation, free download ID4664388 What Is Line And Band Spectra The shapes of these emission spectra fall into two broad types: Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist. What Is Line And Band Spectra.
From classnotes.org.in
Absorption and Emission Spectra Chemistry, Class 11, Structure of Atom What Is Line And Band Spectra Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g.,. What Is Line And Band Spectra.
From www.pinterest.com
Image of absorption, emission, and continuous spectra. Absorption What Is Line And Band Spectra Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. In physics, a ‘line spectrum’ is a set of discrete wavelengths from. What Is Line And Band Spectra.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room What Is Line And Band Spectra Band spectra, or molecular spectra, are produced by molecules radiating. These discrete lines arise due to the. Although objects at high temperature emit a continuous spectrum of electromagnetic radiation, a different kind of spectrum is observed when pure samples of individual elements. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. Line spectrum refers to discrete set. What Is Line And Band Spectra.
From wisc.pb.unizin.org
Emission Spectra and H Atom Levels (M7Q3) UWMadison Chemistry 103/ What Is Line And Band Spectra Band spectra, or molecular spectra, are produced by molecules radiating. The shapes of these emission spectra fall into two broad types: Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. The result is. What Is Line And Band Spectra.
From www.youtube.com
What is the Difference Between Continuous Spectrum and Line Spectrum What Is Line And Band Spectra Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. In. What Is Line And Band Spectra.
From www.youtube.com
What is band spectra YouTube What Is Line And Band Spectra The shapes of these emission spectra fall into two broad types: In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. Band spectra, or molecular spectra, are produced by molecules radiating. Line spectra are also called atomic spectra because the lines. What Is Line And Band Spectra.
From infinitylearn.com
Line spectrum contains information about What Is Line And Band Spectra Band spectra is the name given to groups of lines so closely spaced that each group appears to be a band—e.g., nitrogen spectrum. These discrete lines arise due to the. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. A. What Is Line And Band Spectra.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download What Is Line And Band Spectra Band spectra, or molecular spectra, are produced by molecules radiating. These discrete lines arise due to the. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. The shapes of these emission spectra fall into two broad types: Although objects at. What Is Line And Band Spectra.
From www.researchgate.net
Characteristic lines and bands in the luminescence spectra for diamonds What Is Line And Band Spectra The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Band spectra, or molecular spectra, are produced by molecules radiating. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. These discrete lines arise due to the. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from. What Is Line And Band Spectra.
From www.researchgate.net
(a) Visible lines and bands as a function of time on a linear scale What Is Line And Band Spectra A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Band spectra. What Is Line And Band Spectra.
From chem.libretexts.org
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts What Is Line And Band Spectra These discrete lines arise due to the. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Band spectra is the name given to groups of lines so closely spaced that. What Is Line And Band Spectra.
From www.esa.int
ESA Absorption and emission spectra of various elements What Is Line And Band Spectra Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Band spectra, or molecular spectra, are produced by molecules radiating. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a. What Is Line And Band Spectra.
From www.slideserve.com
PPT What is Light? PowerPoint Presentation, free download ID4664388 What Is Line And Band Spectra Band spectra, or molecular spectra, are produced by molecules radiating. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. These discrete lines arise due to the. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Band spectra is the name given to groups of lines so closely spaced that. What Is Line And Band Spectra.
From lessonliblekythoses.z21.web.core.windows.net
Types Of Spectra Worksheet What Is Line And Band Spectra In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. A line spectrum appears as a series of individual lines, each representing a particular. What Is Line And Band Spectra.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b What Is Line And Band Spectra These discrete lines arise due to the. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Band spectra, or molecular spectra, are produced by molecules radiating. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms,. What Is Line And Band Spectra.
From www.pinterest.com
Absorption spectrum of the elements spectroscopy Physics, Earth and What Is Line And Band Spectra Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. A line spectrum appears as a series of individual lines, each representing a particular wavelength at which light is emitted or absorbed. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. Although objects. What Is Line And Band Spectra.
From www.researchgate.net
Maximum intensity of several spectral lines and bands as function of What Is Line And Band Spectra Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. The shapes of these emission spectra fall into two broad types: These. What Is Line And Band Spectra.
From www.slideserve.com
PPT Electron Arrangement PowerPoint Presentation ID1598428 What Is Line And Band Spectra In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. These discrete. What Is Line And Band Spectra.
From www.researchgate.net
Thermal imager spectral band selection criteria (a) Thermal image What Is Line And Band Spectra Line spectrum refers to discrete set of frequencies emitted or absorbed by atoms, seen as distinct lines. These discrete lines arise due to the. Band spectra, or molecular spectra, are produced by molecules radiating. Line spectra are also called atomic spectra because the lines represent wavelengths radiated from atoms when electrons change from. The shapes of these emission spectra fall. What Is Line And Band Spectra.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b What Is Line And Band Spectra The result is an emission spectrum that shows the intensity of emission as a function of wavelength. In physics, a ‘line spectrum’ is a set of discrete wavelengths from gaseous atoms, whereas a ‘band spectrum’ is produced by molecules, which consist of a series of closely. Band spectrum refers to continuous spectrum with bands, typical of molecules, due. A line. What Is Line And Band Spectra.