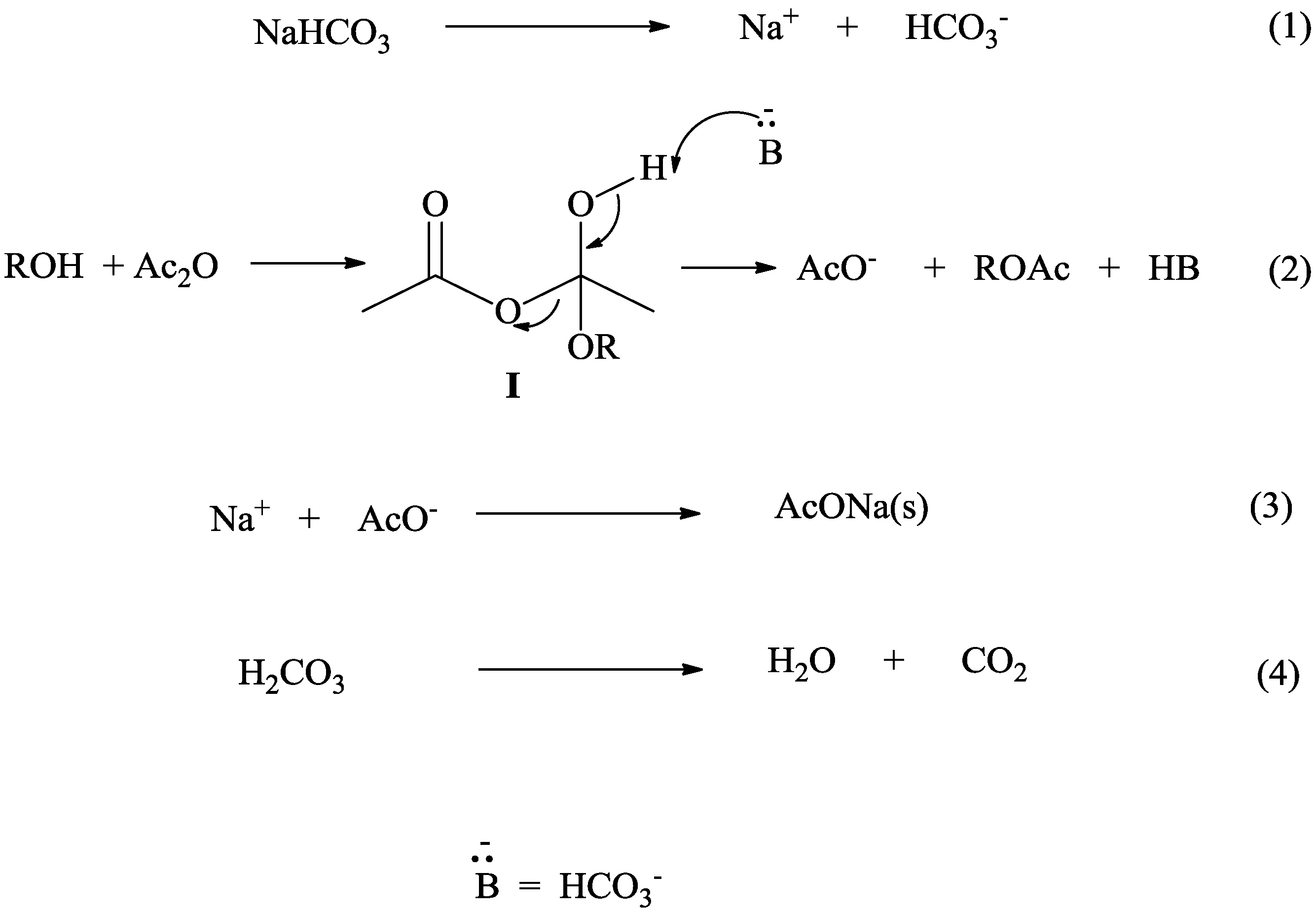
Acetic Acid + Nahco3 . The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Nahco 3 + hc 2. Reaction of nahco 3 and acetic acid: These 2 components react in. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Nahco3 + ch3cooh → co2 + h2o +. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The molecular formula of the acetic acid is \[c{h_3}cooh\]. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Nahco 3 + ch 3.
from ar.inspiredpencil.com
Nahco 3 + ch 3. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Reaction of nahco 3 and acetic acid: These 2 components react in. The molecular formula of the acetic acid is \[c{h_3}cooh\]. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Nahco3 + ch3cooh → co2 + h2o +.
Sodium Bicarbonate And Acetic Acid
Acetic Acid + Nahco3 These 2 components react in. Nahco 3 + ch 3. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Reaction of nahco 3 and acetic acid: First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. Nahco 3 + hc 2. Nahco3 + ch3cooh → co2 + h2o +. The molecular formula of the acetic acid is \[c{h_3}cooh\].
From thomasyouthhouse.blogspot.com
Sodium Bicarbonate and Acetic Acid Balanced Chemical Equation Acetic Acid + Nahco3 First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco 3 + ch 3. The chemical. Acetic Acid + Nahco3.
From daniels-experiments.blogspot.com
Daniel's Experiments Volcano Experiment Acetic Acid + Nahco3 These 2 components react in. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Nahco3 + ch3cooh → co2 + h2o +. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Reaction of nahco 3. Acetic Acid + Nahco3.
From byjus.com
Which of the following reacts is not shown by formic acid? Reaction Acetic Acid + Nahco3 These 2 components react in. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco 3 + ch 3. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: Nahco3 + ch3cooh → co2 + h2o +. The molecular formula of the acetic acid is \[c{h_3}cooh\]. Nahco 3 +. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED (1) Acetic acid is a (circle one) weak acid 13 (8) Write the Acetic Acid + Nahco3 The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written:. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Write the balanced equation for the reaction of acetic acid Acetic Acid + Nahco3 The molecular formula of the acetic acid is \[c{h_3}cooh\]. Nahco 3 + hc 2. Reaction of nahco 3 and acetic acid: The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: These 2 components react in. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to. Acetic Acid + Nahco3.
From www.youtube.com
acetic acid (vinegar) + sodiam bicarbonate (baking soda)_______ NaHCO3 Acetic Acid + Nahco3 Nahco3 + ch3cooh → co2 + h2o +. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: The chemical equation for the reaction between sodium bicarbonate and acetic acid. Acetic Acid + Nahco3.
From en-academic.com
Acetic acid Acetic Acid + Nahco3 When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Nahco 3 + hc 2. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: The molecular formula of the acetic acid is \[c{h_3}cooh\]. The chemical equation for the reaction between sodium. Acetic Acid + Nahco3.
From ar.inspiredpencil.com
Sodium Bicarbonate And Acetic Acid Acetic Acid + Nahco3 Nahco 3 + hc 2. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Nahco 3 + ch 3. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco3 + ch3cooh → co2 + h2o. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED You want to help your little brother make an exploding volcano Acetic Acid + Nahco3 Nahco 3 + ch 3. Nahco3 + ch3cooh → co2 + h2o +. Nahco 3 + hc 2. These 2 components react in. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar. Acetic Acid + Nahco3.
From cymitquimica.com
Acetic Acid CymitQuimica Acetic Acid + Nahco3 The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: When sodium bicarbonate nahco 3. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Consider the balanced chemical equation for the production of Acetic Acid + Nahco3 These 2 components react in. Nahco 3 + ch 3. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Nahco 3 + hc 2. The molecular formula of the acetic acid is \[c{h_3}cooh\]. Nahco3 + ch3cooh → co2 + h2o +.. Acetic Acid + Nahco3.
From slideplayer.com
DISTINGUISHING TEST BETWEEN ORGANIC COMPOUNDS ppt video online download Acetic Acid + Nahco3 The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco 3 + hc 2. Reaction of nahco 3 and acetic acid: First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: The molecular formula of the acetic acid is. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Question 38 (2 points) Which is primary standard base? NaHCO3 Acetic Acid + Nahco3 These 2 components react in. Nahco3 + ch3cooh → co2 + h2o +. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: The molecular formula of the acetic acid. Acetic Acid + Nahco3.
From www.coursehero.com
[Solved] Inall 2 Mass of acetic acid Density of acetic acid 1.00 g/mL Acetic Acid + Nahco3 First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Nahco 3 + hc 2. These 2 components react in. Nahco3 + ch3cooh → co2 + h2o +. Nahco 3 + ch 3. The molecular formula of the acetic acid is \[c{h_3}cooh\]. Reaction of nahco. Acetic Acid + Nahco3.
From studylib.net
NaHCO3(s) + CH3COOH(aq) → CO2(g) + CH3COONa(aq) + H2O(l) Acetic Acid + Nahco3 First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Nahco 3 + hc 2. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco 3 + ch 3. Baking soda is a powdered chemical compound called sodium bicarbonate,. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Baking soda and vinegar react according to the following Acetic Acid + Nahco3 The molecular formula of the acetic acid is \[c{h_3}cooh\]. Nahco 3 + ch 3. Nahco 3 + hc 2. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: First, there is a double displacement reaction in which acetic acid. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Aqueous solutions of sodium bicarbonate and sulfuric acid react Acetic Acid + Nahco3 Nahco 3 + ch 3. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: The molecular formula of the acetic acid is \[c{h_3}cooh\]. Nahco3 + ch3cooh → co2 + h2o +. First, there is a double displacement reaction in. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED The chemical equation for the reaction of baking soda (sodium Acetic Acid + Nahco3 The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco 3 + hc 2. Nahco3 + ch3cooh → co2 + h2o +. Reaction of nahco 3 and acetic acid: Nahco 3 + ch 3. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: The molecular formula of the. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Write a balanced net ionic equation for the reaction of aqueous Acetic Acid + Nahco3 The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco 3 + hc 2. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Nahco3 + ch3cooh → co2 + h2o +. The molecular formula of the acetic acid is \[c{h_3}cooh\]. These 2 components react in. Reaction of nahco. Acetic Acid + Nahco3.
From www.teachoo.com
[Carbon] Ethanoic acid Formation, Properties, Uses [with Reactions] Acetic Acid + Nahco3 When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. These 2 components react in. Reaction of nahco 3 and acetic acid: Nahco3 + ch3cooh → co2 + h2o +. Nahco 3 + hc 2. The molecular formula of the acetic acid is \[c{h_3}cooh\]. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes. Acetic Acid + Nahco3.
From www.coursehero.com
[Solved] Write balanced chemical equation for the neutralisation of Acetic Acid + Nahco3 Nahco 3 + ch 3. Nahco 3 + hc 2. The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Reaction of nahco 3 and acetic acid: The chemical reaction. Acetic Acid + Nahco3.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid + Nahco3 The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco 3 + ch 3. The molecular formula of the acetic acid is \[c{h_3}cooh\]. Nahco 3 + hc 2. Nahco3 + ch3cooh → co2 + h2o +. These 2 components react in. The chemical equation for the reaction between sodium bicarbonate and acetic acid is. Acetic Acid + Nahco3.
From www.youtube.com
Acetic acid (ethanoic acid) and Sodium hydroxide reaction CH3COOH Acetic Acid + Nahco3 Nahco3 + ch3cooh → co2 + h2o +. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The molecular formula of the acetic acid is \[c{h_3}cooh\]. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Reaction of nahco 3 and acetic acid: The chemical equation for the reaction. Acetic Acid + Nahco3.
From www.teachoo.com
[MCQ] Sodium hydrogen carbonate when added to acetic acid evolves gas Acetic Acid + Nahco3 First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Nahco3 + ch3cooh → co2 + h2o. Acetic Acid + Nahco3.
From www.chegg.com
Solved 3. CH COOH+ NaOH acetic acid 4. CH3COOH +NaHCO3 Acetic Acid + Nahco3 Reaction of nahco 3 and acetic acid: These 2 components react in. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. First, there is a double. Acetic Acid + Nahco3.
From www.youtube.com
Acetic Acid (CH3COOH) 3D Model with Lewis Structure YouTube Acetic Acid + Nahco3 Nahco3 + ch3cooh → co2 + h2o +. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: These 2 components react in. The molecular formula of the acetic acid is \[c{h_3}cooh\]. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes. Acetic Acid + Nahco3.
From www.youtube.com
Is NaHCO3 acidic, basic, or neutral (in water)? YouTube Acetic Acid + Nahco3 Nahco3 + ch3cooh → co2 + h2o +. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. The molecular formula of the acetic acid is \[c{h_3}cooh\]. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Reaction of nahco 3 and acetic acid: The chemical equation for the reaction between sodium. Acetic Acid + Nahco3.
From pdfprof.com
acetylation of phenol using acetic anhydride Acetic Acid + Nahco3 The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: These 2 components react in. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. The molecular formula of the acetic acid is \[c{h_3}cooh\]. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. First, there is. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Test Reaction with the Solvent Solubility Compound Distilled Acetic Acid + Nahco3 The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Baking soda is a powdered chemical compound. Acetic Acid + Nahco3.
From www.chegg.com
Solved Reaction 1 Acetic acid and sodium bicarbonate Acetic Acid + Nahco3 Reaction of nahco 3 and acetic acid: The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: These 2 components react in. Nahco 3 + ch 3. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes.. Acetic Acid + Nahco3.
From www.pdfprof.com
acidity of benzoic acid and acetic acid Acetic Acid + Nahco3 The chemical equation for the reaction between sodium bicarbonate and acetic acid is as follows: The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: Nahco3 + ch3cooh → co2 + h2o +. Nahco 3 + ch 3. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium. Acetic Acid + Nahco3.
From www.numerade.com
SOLVED Consider the titration of acetic acid (CH3COOH) in vinegar with Acetic Acid + Nahco3 Nahco 3 + hc 2. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Reaction of nahco 3 and acetic acid: Nahco 3 + ch 3. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Nahco3 + ch3cooh. Acetic Acid + Nahco3.
From www.youtube.com
Type of Reaction for NaHCO3 = Na2CO3 + H2O + CO2 YouTube Acetic Acid + Nahco3 The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. Reaction of nahco 3 and acetic acid: Nahco 3 + ch 3. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. Nahco3 + ch3cooh → co2. Acetic Acid + Nahco3.
From www.coursehero.com
[Solved] Draw the reaction between acetylsalicylic acid and NaHCO 3 Acetic Acid + Nahco3 These 2 components react in. First, there is a double displacement reaction in which acetic acid in the vinegar reacts with sodium bicarbonate to form sodium acetate and carbonic acid: Nahco 3 + hc 2. Baking soda is a powdered chemical compound called sodium bicarbonate, and vinegar includes acetic acid. The chemical reaction between baking soda (sodium bicarbonate) and vinegar. Acetic Acid + Nahco3.
From www.dreamstime.com
3D Image of Acetic Acid Skeletal Formula Stock Illustration Acetic Acid + Nahco3 Nahco 3 + hc 2. These 2 components react in. When sodium bicarbonate nahco 3 reacts with acetic acid, a neutralization reaction takes. The chemical reaction between baking soda (sodium bicarbonate) and vinegar (acetic acid) can be written: The molecular formula of the acetic acid is \[c{h_3}cooh\]. Reaction of nahco 3 and acetic acid: First, there is a double displacement. Acetic Acid + Nahco3.