The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To . Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. We have to find the. The orbitals are solutions of the quantum mechanical equation and are energy levels. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. So a photon can be absorbed by an atom. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another.
from www.numerade.com
Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. We have to find the. The orbitals are solutions of the quantum mechanical equation and are energy levels. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. So a photon can be absorbed by an atom. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon).
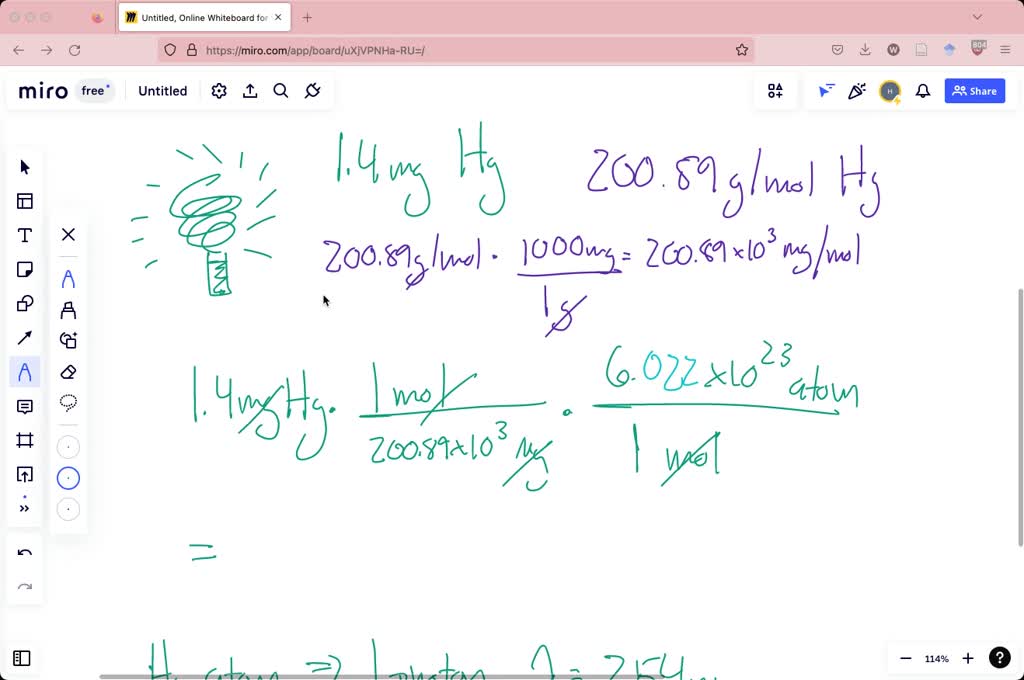
A modern compact fluorescent lamp contains 1.4 mg of mercury. If each
The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). So a photon can be absorbed by an atom. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. The orbitals are solutions of the quantum mechanical equation and are energy levels. We have to find the. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom.
From www.chegg.com
Solved As a mercury atom absorbs a photon of energy, an The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. So a photon can be absorbed by an atom. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom.. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From chemcollective.org
CHEM1315 Lab 8 Atomic Spectrum The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). We have to find the. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. So a photon can be absorbed by an atom. The orbitals are solutions of the quantum mechanical equation and are energy. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From chem.libretexts.org
7.1 Vocabulary Chemistry LibreTexts The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. So a photon can be absorbed by an atom. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Radiation from a mercury source will not be absorbed however, because its emitted photons have. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.chegg.com
Solved shows a few energy levels of the mercury atom. a. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To It is given that, wavelength of photon, it strikes a mercury atom in the ground state. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. So a photon can be absorbed by an atom. In the photoelectric absorption process, a photon undergoes an interaction with an absorber. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.vrogue.co
Orbit Levels Of Electrons In An Atom vrogue.co The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To So a photon can be absorbed by an atom. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). It is given that, wavelength of photon, it strikes a mercury atom in the ground state. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From manualdbemory.z13.web.core.windows.net
Schematic Energy Diagram Of An Atom The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. We have to. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.alamy.com
Fundamental particle Cut Out Stock Images & Pictures Alamy The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. So a photon can be absorbed by an atom. Radiation from. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.nagwa.com
Lesson Electron Energy Level Transitions Nagwa The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. So a photon can be absorbed by an atom. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. Fluorescence occurs when an electron in. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From studylib.net
Absorption / Emission of Photons and Conservation of Energy The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.nagwa.com
Question Video Finding the Required Wavelength of an Absorbed Photon The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. We have to find the. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. Electrons can transfer to. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.chegg.com
Solved Electrically excited mercury atoms have particularly The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To We have to find the. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). The orbitals are solutions of the quantum mechanical equation and are energy levels. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. In the. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To We have to find the. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. The orbitals are solutions of the quantum mechanical equation and are energy levels. Electrons can transfer to a higher. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.numerade.com
SOLVED The figure shows the energy level diagram of a mercury atom. A The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. We have to find the. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. It is given that, wavelength of photon, it strikes a mercury atom. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From chemicalengineeringworld.com
Mercury Element Properties and Information Chemical Engineering World The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. We have to find the. So a photon can be absorbed by an atom. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Electrons can transfer to a higher energy level by absorbing energy. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To So a photon can be absorbed by an atom. We have to find the. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Electrons can transfer. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.numerade.com
SOLVEDRefer to Figure 2821 for Problems 49 and 50. A mercury atom in The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. The orbitals are solutions of the quantum mechanical equation and are energy levels. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.researchgate.net
Energy level diagram of mercury atom. Download Scientific Diagram The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. So a photon can be absorbed by an atom. We have to find the. Radiation from a. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.numerade.com
A modern compact fluorescent lamp contains 1.4 mg of mercury. If each The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To So a photon can be absorbed by an atom. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. In the photoelectric absorption process, a photon undergoes an interaction with an absorber. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.numerade.com
SOLVEDA mercury atom drops in energy from 1.413 ×10^18 J to 1.069 ×10 The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). It is given that, wavelength of photon, it strikes a mercury atom in the ground state. The orbitals are solutions of the quantum mechanical equation and are energy levels. So a photon can be absorbed by an atom. Fluorescence occurs when an. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.numerade.com
SOLVEDMercury Mercury’s atomic emission spectrum is shown in Figure 5. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. So a photon can be absorbed by an atom. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). Fluorescence occurs when an electron in an atomic orbital absorbs energy from. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.chemistrylearner.com
Mercury Definition, Facts, Symbol, Discovery, Property, Uses The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). It is given that, wavelength of photon, it strikes a mercury atom in the ground state. The orbitals are solutions of the quantum mechanical equation and are energy levels. We have to find the. Radiation from a mercury source will not be. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From chemistrypuns-periodically.weebly.com
Chemistry Electron Emission Spectrum The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). So a photon can be absorbed by an atom. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. We have to find the. Radiation from a mercury source will not be absorbed however, because its emitted photons. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From exogxahpg.blob.core.windows.net
What Is The Energy (In Joules) Of One Quantum Of This Radiation at The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. The orbitals are solutions of the quantum mechanical equation and are energy levels. So a photon can be absorbed by an atom. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. Fluorescence occurs when. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From circuitestrilhatc6.z13.web.core.windows.net
Diagram Of Hydrogen Atom The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. We have to find the. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. Electrons can transfer to a higher. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.youtube.com
What is the wavelength of light emitted when the electron in a hydrogen The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). We have to find the. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. So a photon can be absorbed by an atom. It is given that, wavelength of photon,. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From app.emaze.com
Waves, Photons and Matter on emaze The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). So a photon can be absorbed by an atom. The orbitals are solutions of the quantum mechanical equation and are energy levels. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From users.highland.edu
Atomic Spectra and Models of the Atom The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To It is given that, wavelength of photon, it strikes a mercury atom in the ground state. We have to find the. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Electrons can transfer. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.toppr.com
An electron passing through a potential difference of 4.9 V collides The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. So a photon can be absorbed by an atom. The orbitals are solutions of the quantum mechanical equation and are energy levels. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. It is given that,. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.nachi.org
Atomic Valence Shells Energy Emittance Inspection Gallery InterNACHI® The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To We have to find the. So a photon can be absorbed by an atom. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. The orbitals are solutions of the quantum mechanical equation and are energy levels. Radiation. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From chem.libretexts.org
Chapter 2.3 Atomic Spectra and Models of the Atom Chemistry LibreTexts The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To We have to find the. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). It is given that, wavelength of photon, it strikes a mercury atom in the ground state. Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the.. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From ar.inspiredpencil.com
Electron Energy Levels Photon The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To So a photon can be absorbed by an atom. The orbitals are solutions of the quantum mechanical equation and are energy levels. We have to find the. Electrons can transfer to a higher energy level by absorbing energy (usually in the form of a photon). Radiation from a mercury source will not be absorbed however, because its emitted photons have. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From spiff.rit.edu
Deep inside some stars,the conditions temperature, pressure The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. The orbitals are solutions of the quantum mechanical equation and are energy levels. Fluorescence occurs when an electron in an atomic orbital absorbs energy from. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To Radiation from a mercury source will not be absorbed however, because its emitted photons have frequencies that don't correspond to the. In the photoelectric absorption process, a photon undergoes an interaction with an absorber atom. So a photon can be absorbed by an atom. It is given that, wavelength of photon, it strikes a mercury atom in the ground state.. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.
From www.youtube.com
What is the wavelength of light emitted when the electron in a hydrogen The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To The orbitals are solutions of the quantum mechanical equation and are energy levels. It is given that, wavelength of photon, it strikes a mercury atom in the ground state. So a photon can be absorbed by an atom. Fluorescence occurs when an electron in an atomic orbital absorbs energy from an interaction with a photon or a collision with another.. The Photon Can Be Absorbed By The Mercury Atom Because It Will Accommodate An Energy Level Jump To.