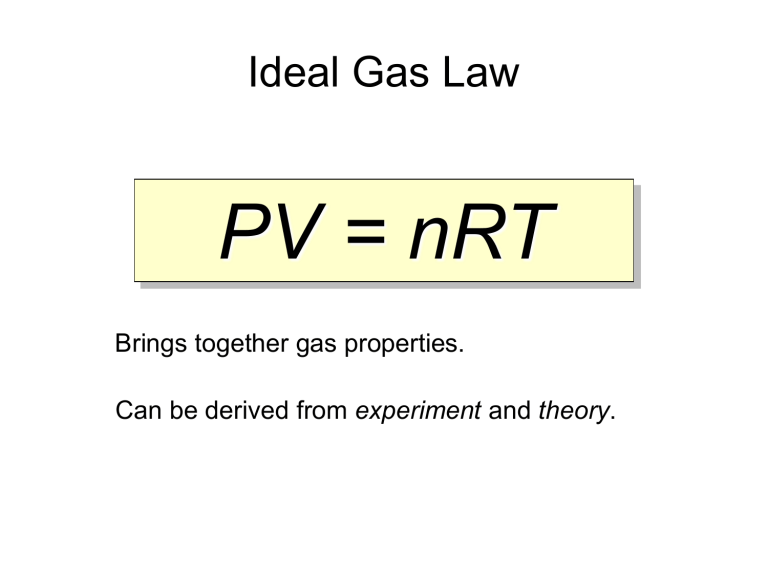
What Is An Ideal Gas Law . Although the law describes the behavior of an. The ideal gas law is the. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move.
from studylib.net
An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Although the law describes the behavior of an. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The ideal gas law is the. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin.
Ideal Gas Law
What Is An Ideal Gas Law The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. Although the law describes the behavior of an. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas law is the. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws.
From www.slideserve.com
PPT The Combined and Ideal Gas Laws PowerPoint Presentation, free What Is An Ideal Gas Law The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. Although the law describes the behavior of an. The ideal gas law is the. Ideal gas, a gas that conforms, in physical. What Is An Ideal Gas Law.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net What Is An Ideal Gas Law The ideal gas law is the. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Although the law describes the behavior of an. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized. What Is An Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 What Is An Ideal Gas Law Although the law describes the behavior of an. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the. What Is An Ideal Gas Law.
From www.thoughtco.com
An Explanation of the Ideal Gas Law What Is An Ideal Gas Law The ideal gas law is the. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. The ideal. What Is An Ideal Gas Law.
From scienceinfo.com
Ideal Gas Equation Ideal Gas and Laws What Is An Ideal Gas Law The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. Although the law describes the behavior of an. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. An ideal gas is a theoretical gas composed of many randomly moving point particles. What Is An Ideal Gas Law.
From www.youtube.com
1.3 Ideal gas equation YouTube What Is An Ideal Gas Law Although the law describes the behavior of an. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. The volume (v) occupied by n moles. What Is An Ideal Gas Law.
From www.bartleby.com
Ideal and Real Gases bartleby What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Although the law describes the behavior of an. The ideal gas law is the. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle. What Is An Ideal Gas Law.
From erebusryling.blogspot.com
Ideal Gas Law R Values PPT Gas Laws PowerPoint Presentation ID What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Although the law describes the behavior of an. An ideal gas is. What Is An Ideal Gas Law.
From www.youtube.com
IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems What Is An Ideal Gas Law Although the law describes the behavior of an. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Ideal gas law, relation between the pressure p, volume v, and temperature. What Is An Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Is An Ideal Gas Law An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The ideal gas law is the.. What Is An Ideal Gas Law.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID5758540 What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas law is the equation of state for an ideal gas. What Is An Ideal Gas Law.
From mmerevise.co.uk
The Ideal Gas Equation MME What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The. What Is An Ideal Gas Law.
From www.slideserve.com
PPT The ideal gas law PowerPoint Presentation, free download ID3608170 What Is An Ideal Gas Law An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and. What Is An Ideal Gas Law.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 What Is An Ideal Gas Law The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. The ideal gas law is the. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Although the law describes the behavior of an. The ideal gas law is the. What Is An Ideal Gas Law.
From mavink.com
Derive Ideal Gas Equation What Is An Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Although the law describes the behavior of an. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature. What Is An Ideal Gas Law.
From sciencenotes.org
Real Gas vs Ideal Gas What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. An. What Is An Ideal Gas Law.
From www.chemistrylearner.com
Ideal Gas Law Statement, Characteristics, Formula & Problems What Is An Ideal Gas Law The ideal gas law is the. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized. What Is An Ideal Gas Law.
From www.youtube.com
The Ideal Gas Law YouTube What Is An Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Although the law describes the behavior of an. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws.. What Is An Ideal Gas Law.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation ID210554 What Is An Ideal Gas Law Although the law describes the behavior of an. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. The ideal gas law is the. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law,. What Is An Ideal Gas Law.
From gaspinsami.blogspot.com
Gas What Is The Ideal Gas Law What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Although the law describes the behavior of an. The ideal gas law is. What Is An Ideal Gas Law.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube What Is An Ideal Gas Law The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t). What Is An Ideal Gas Law.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. An ideal gas is a theoretical gas composed of many randomly moving. What Is An Ideal Gas Law.
From sciencenotes.org
Ideal Gas Law Formula and Examples What Is An Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Although the law describes the behavior of an. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature. What Is An Ideal Gas Law.
From studylib.net
Ideal Gas Law What Is An Ideal Gas Law The ideal gas law is the. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. An ideal gas is a theoretical gas composed of many randomly moving point. What Is An Ideal Gas Law.
From atomstalk.com
The Gas Laws AtomsTalk What Is An Ideal Gas Law The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The ideal gas law is. What Is An Ideal Gas Law.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint What Is An Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The ideal gas law is the. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions.. What Is An Ideal Gas Law.
From mungfali.com
Ideal Gas Law Units 7EC What Is An Ideal Gas Law Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. Ideal gas law, relation between the pressure p, volume v, and temperature t of a. What Is An Ideal Gas Law.
From gaspinsami.blogspot.com
Gas What Is The Ideal Gas Law What Is An Ideal Gas Law Although the law describes the behavior of an. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. The ideal gas law is the. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures,. What Is An Ideal Gas Law.
From www.logiota.com
Deviation from Ideal Gas Behavior States of Matter Physical What Is An Ideal Gas Law The ideal gas law is the. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. The ideal. What Is An Ideal Gas Law.
From www.slideserve.com
PPT using The Ideal Gas Law PowerPoint Presentation, free download What Is An Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Although. What Is An Ideal Gas Law.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii What Is An Ideal Gas Law Ideal gas law, relation between the pressure p, volume v, and temperature t of a gas in the limit of low pressures and high temperatures, such that the molecules of the gas move. Although the law describes the behavior of an. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to. What Is An Ideal Gas Law.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy What Is An Ideal Gas Law The ideal gas law is the. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas law, relation between the. What Is An Ideal Gas Law.
From www.sliderbase.com
The Ideal Gas Law Presentation Chemistry What Is An Ideal Gas Law The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. The ideal gas law is a combination of simpler gas laws such as boyle's, charles's, avogadro's and amonton's laws. The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin.. What Is An Ideal Gas Law.
From mungfali.com
Ideal Gas Law Units 7EC What Is An Ideal Gas Law Although the law describes the behavior of an. The ideal gas law is the equation of state for an ideal gas that relates pressure, volume, gas quantity, and absolute temperature. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is. What Is An Ideal Gas Law.
From gaspinsami.blogspot.com
Gas Ideal Gas Law What Is An Ideal Gas Law The volume (v) occupied by n moles of any gas has a pressure (p) at temperature (t) in kelvin. The ideal gas law is the. An ideal gas is a theoretical gas composed of many randomly moving point particles that are not subject to interparticle interactions. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation. What Is An Ideal Gas Law.