Why Is Ethanol A Bad Solvent For Extraction . Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. The efficacy of ethanol as a solvent for some. It can also form hydrogen. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Extraction means drawing a compound out of a mixture using a solvent. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. It means compounds have a choice of two solvents that they can. Solvent partitioning is more specific. The ethanol extraction process generally has. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. This process leverages the solvent properties.
from encyclopedia.pub
Solvent partitioning is more specific. The ethanol extraction process generally has. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. It can also form hydrogen. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Extraction means drawing a compound out of a mixture using a solvent. This process leverages the solvent properties. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. It means compounds have a choice of two solvents that they can. Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction:
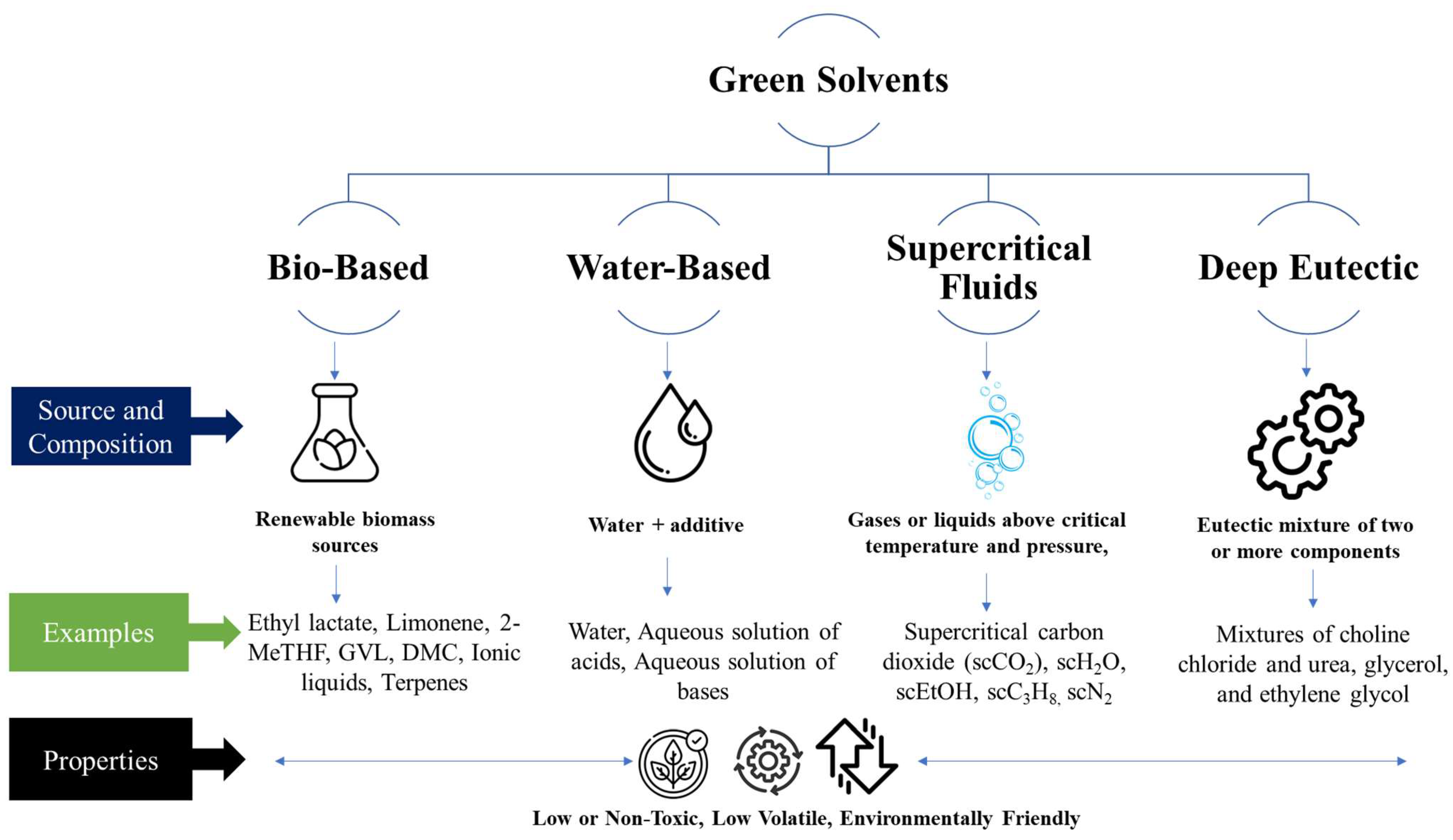
Classification of Green Solvents Encyclopedia MDPI
Why Is Ethanol A Bad Solvent For Extraction Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. The ethanol extraction process generally has. Solvent partitioning is more specific. Extraction means drawing a compound out of a mixture using a solvent. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. The efficacy of ethanol as a solvent for some. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: This process leverages the solvent properties. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. It means compounds have a choice of two solvents that they can. It can also form hydrogen.
From fairnorganic.com
EXTRACTION METHODS Why Is Ethanol A Bad Solvent For Extraction It means compounds have a choice of two solvents that they can. This process leverages the solvent properties. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Solvent partitioning is more specific. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. The ethanol. Why Is Ethanol A Bad Solvent For Extraction.
From blog.gotopac.com
What's the Best Ethanol for Commercial Solvent Extraction? FCC, USP, HPLC Why Is Ethanol A Bad Solvent For Extraction It can also form hydrogen. Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. This process leverages the solvent properties. Extraction means drawing a compound out of a mixture using a. Why Is Ethanol A Bad Solvent For Extraction.
From www.edenlabs.com
Ethanol Extraction Process Diagram Eden Labs Why Is Ethanol A Bad Solvent For Extraction This process leverages the solvent properties. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Extraction means drawing a compound out of a mixture using a solvent. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. The efficacy of ethanol. Why Is Ethanol A Bad Solvent For Extraction.
From www.maratek.com
Cryogenic Ethanol Extraction Equipment for Extracting Cannabis Oil Why Is Ethanol A Bad Solvent For Extraction The efficacy of ethanol as a solvent for some. Solvent partitioning is more specific. This process leverages the solvent properties. Extraction means drawing a compound out of a mixture using a solvent. It can also form hydrogen. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of. Why Is Ethanol A Bad Solvent For Extraction.
From cedarstoneindustry.com
Why Ethanol Is the Best Solvent for Extraction Why Is Ethanol A Bad Solvent For Extraction Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. It can also form hydrogen. It means compounds have a choice of two solvents that they can. Here are some. Why Is Ethanol A Bad Solvent For Extraction.
From c1d1labs.org
C1D1 Labs Ethanol Extraction Equipment Why Is Ethanol A Bad Solvent For Extraction The ethanol extraction process generally has. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. Extraction means drawing a compound out of a mixture using a solvent. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. The efficacy of ethanol. Why Is Ethanol A Bad Solvent For Extraction.
From eduinput.com
Why Is Ethanol Good Solvent For Extraction? Why Is Ethanol A Bad Solvent For Extraction Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. It can also form hydrogen. Solvent partitioning is more specific. The ethanol extraction process generally has. This process leverages the solvent properties. Ethanol is emerging as one. Why Is Ethanol A Bad Solvent For Extraction.
From www.youtube.com
mr i explains The Distillation of Ethanol YouTube Why Is Ethanol A Bad Solvent For Extraction It means compounds have a choice of two solvents that they can. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. The efficacy of ethanol as a solvent for some. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide. Why Is Ethanol A Bad Solvent For Extraction.
From simplesolvents.com
Solvent Extraction 101 Techniques, Applications, & Solvents Why Is Ethanol A Bad Solvent For Extraction In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. The ethanol extraction process generally has. This process leverages the solvent properties. The efficacy of ethanol as a solvent for some. It can also form hydrogen. Ethanol extraction is a versatile technique used to extract valuable compounds from plant. Why Is Ethanol A Bad Solvent For Extraction.
From www.edenlabs.com
Ethanol Solvent Recovery Diagram Eden Labs Why Is Ethanol A Bad Solvent For Extraction In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. The efficacy of ethanol as a solvent for some. Extraction means drawing a compound out of a mixture using a solvent. The ethanol extraction process generally has. It means compounds have a choice of two solvents that they can.. Why Is Ethanol A Bad Solvent For Extraction.
From extractiongradesolvents.com
What Are Ethanol Extraction Methods? ExtractionGradeSolvents Why Is Ethanol A Bad Solvent For Extraction Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. The efficacy of ethanol as a solvent for some. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. Solvent partitioning is more specific. It means compounds have a choice. Why Is Ethanol A Bad Solvent For Extraction.
From www.youtube.com
DISTILLATION OF WATER AND ETHANOL YouTube Why Is Ethanol A Bad Solvent For Extraction The ethanol extraction process generally has. Solvent partitioning is more specific. It means compounds have a choice of two solvents that they can. The efficacy of ethanol as a solvent for some. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. Even with today’s technological advancements in. Why Is Ethanol A Bad Solvent For Extraction.
From extractiongradesolvents.com
Ethanol Use In Plant Extraction ExtractionGradeSolvents Why Is Ethanol A Bad Solvent For Extraction Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. Solvent partitioning is more specific. In this comprehensive guide, we will delve into different. Why Is Ethanol A Bad Solvent For Extraction.
From extractiongradesolvents.com
Ethanol Solvent for Extraction? ExtractionGradeSolvents Why Is Ethanol A Bad Solvent For Extraction This process leverages the solvent properties. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. The ethanol extraction process generally has. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. Here are some key chemical properties of ethanol that make it an. Why Is Ethanol A Bad Solvent For Extraction.
From www.cedarstoneindustry.com
Ethanol Extraction, Cannabis and CBD Extraction Equipment Why Is Ethanol A Bad Solvent For Extraction This process leverages the solvent properties. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. The efficacy of ethanol as a solvent for some. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety.. Why Is Ethanol A Bad Solvent For Extraction.
From www.edenlabs.com
High Performance Commercial Ethanol Extraction Eden Labs Why Is Ethanol A Bad Solvent For Extraction It can also form hydrogen. Extraction means drawing a compound out of a mixture using a solvent. This process leverages the solvent properties. Solvent partitioning is more specific. The efficacy of ethanol as a solvent for some. It means compounds have a choice of two solvents that they can. The ethanol extraction process generally has. Even with today’s technological advancements. Why Is Ethanol A Bad Solvent For Extraction.
From www.chemicals.co.uk
How is Ethanol Converted into Ethanoic Acid? Why Is Ethanol A Bad Solvent For Extraction The ethanol extraction process generally has. Solvent partitioning is more specific. This process leverages the solvent properties. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. The efficacy of ethanol as a solvent for some. Extraction means drawing a compound out of a mixture using a solvent. Ethanol is emerging as one of the more. Why Is Ethanol A Bad Solvent For Extraction.
From deltaseparations.com
CBD & THC Oil Extraction Equipment Guide for Cannabis and Hemp Why Is Ethanol A Bad Solvent For Extraction Extraction means drawing a compound out of a mixture using a solvent. Solvent partitioning is more specific. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. The efficacy of ethanol as a solvent for some. It can also form hydrogen. In this comprehensive guide, we will delve. Why Is Ethanol A Bad Solvent For Extraction.
From www.vrogue.co
What Is Solvent Extraction And Why Is It Important So vrogue.co Why Is Ethanol A Bad Solvent For Extraction Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: Even with today’s technological advancements. Why Is Ethanol A Bad Solvent For Extraction.
From www.cbgbiotech.com
Ethanol Extraction Systems for Extracting Cannabis Oil CBG Biotech Why Is Ethanol A Bad Solvent For Extraction Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar. Why Is Ethanol A Bad Solvent For Extraction.
From chemistnotes.com
Solvent extraction Principle, easy process, application Chemistry Notes Why Is Ethanol A Bad Solvent For Extraction Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. It can also form hydrogen. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Solvent partitioning is more specific. It means compounds have a choice of two solvents that they can. Ethanol is emerging. Why Is Ethanol A Bad Solvent For Extraction.
From cannabiscentrifuges.com
Safe Ethanol Extraction Via Centrifugation Cannabis Centrifuges Why Is Ethanol A Bad Solvent For Extraction In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. Solvent partitioning is more specific. The efficacy of ethanol as a solvent for some. Ethanol extraction is. Why Is Ethanol A Bad Solvent For Extraction.
From www.youtube.com
Solvent Extraction with Example Chapter 2F.Sc Chemistry Part1 Why Is Ethanol A Bad Solvent For Extraction This process leverages the solvent properties. It can also form hydrogen. Extraction means drawing a compound out of a mixture using a solvent. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. The efficacy of ethanol as a solvent for some. Solvent partitioning is more specific. In. Why Is Ethanol A Bad Solvent For Extraction.
From simplesolvents.com
Methods Of Ethanol Extraction Optimization In Extraction Why Is Ethanol A Bad Solvent For Extraction Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. This process leverages the solvent properties. Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: It can also form hydrogen. Ethanol is emerging as one of the more popular solvents, because it is safe for infused. Why Is Ethanol A Bad Solvent For Extraction.
From cbd.market
CBD Extraction Methods What are the Best and Safest? CBD.market Why Is Ethanol A Bad Solvent For Extraction Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. The efficacy of ethanol as a solvent for some. It means compounds have a choice of two solvents that they can. Ethanol is emerging as one of the more popular. Why Is Ethanol A Bad Solvent For Extraction.
From www.coleparmer.com
Plant Solvent Extraction Method Using Ethanol 3 Steps ColeParmer Why Is Ethanol A Bad Solvent For Extraction The ethanol extraction process generally has. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Extraction means drawing a compound out of a mixture using a solvent. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Even with today’s technological advancements in extraction. Why Is Ethanol A Bad Solvent For Extraction.
From www.27fchileanway.cl
Why is 70 ethanol used for extraction? 27F Chilean Way Why Is Ethanol A Bad Solvent For Extraction Here are some key chemical properties of ethanol that make it an ideal solvent for plant extraction: Extraction means drawing a compound out of a mixture using a solvent. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. Even with today’s technological advancements in extraction. Why Is Ethanol A Bad Solvent For Extraction.
From www.chegg.com
Solved Ethanol can be extracted in a liquidliquid Why Is Ethanol A Bad Solvent For Extraction Solvent partitioning is more specific. This process leverages the solvent properties. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. The efficacy of ethanol as a solvent for some. It can also form hydrogen. Extraction means drawing a compound. Why Is Ethanol A Bad Solvent For Extraction.
From vitalmagazineonline.com
Infographics The Ethanol Process Vital A news & media resource Why Is Ethanol A Bad Solvent For Extraction Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. Solvent partitioning is more specific. The ethanol extraction process generally has. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. Here are some. Why Is Ethanol A Bad Solvent For Extraction.
From www.vrogue.co
What Is Solvent Extraction And Why Is It Important So vrogue.co Why Is Ethanol A Bad Solvent For Extraction Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Solvent partitioning is more specific. This process leverages the solvent properties. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. The efficacy of ethanol as a solvent for some. Extraction means. Why Is Ethanol A Bad Solvent For Extraction.
From future4200.com
Ethanol Azeotropes Attn EtOH cannabis extractors! Solvent Recovery Why Is Ethanol A Bad Solvent For Extraction Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Ethanol is emerging as one of the more popular solvents, because it is safe for infused edibles and compatible with any type of container. The efficacy of ethanol as a solvent for some. Here are some key chemical properties of ethanol that make it an ideal. Why Is Ethanol A Bad Solvent For Extraction.
From ecurrencythailand.com
What Is Extraction Solvent Ethanol? The 13 Latest Answer Why Is Ethanol A Bad Solvent For Extraction It means compounds have a choice of two solvents that they can. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and nonpolar substances. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. The efficacy of ethanol as a solvent for some. Ethanol. Why Is Ethanol A Bad Solvent For Extraction.
From www.researchgate.net
The extraction solution using different solvents; (1) ethanol 1, (2 Why Is Ethanol A Bad Solvent For Extraction Extraction means drawing a compound out of a mixture using a solvent. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. The ethanol extraction process generally has. The efficacy of ethanol as a solvent for some. This process leverages the solvent properties. In this comprehensive guide, we will delve into different ethanol extraction methods, explore. Why Is Ethanol A Bad Solvent For Extraction.
From encyclopedia.pub
Classification of Green Solvents Encyclopedia MDPI Why Is Ethanol A Bad Solvent For Extraction In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Solvent partitioning is more specific. Ethanol extraction is a versatile technique used to extract valuable compounds from plant materials. Ethanol is a good solvent for extraction because it is polar and can dissolve a wide range of polar and. Why Is Ethanol A Bad Solvent For Extraction.
From ecurrencythailand.com
What Is Extraction Solvent Ethanol? The 13 Latest Answer Why Is Ethanol A Bad Solvent For Extraction It means compounds have a choice of two solvents that they can. Even with today’s technological advancements in extraction chemistry, ethanol is the preferred solvent for botanical extraction. In this comprehensive guide, we will delve into different ethanol extraction methods, explore optimization strategies, and provide an overview of safety. Here are some key chemical properties of ethanol that make it. Why Is Ethanol A Bad Solvent For Extraction.