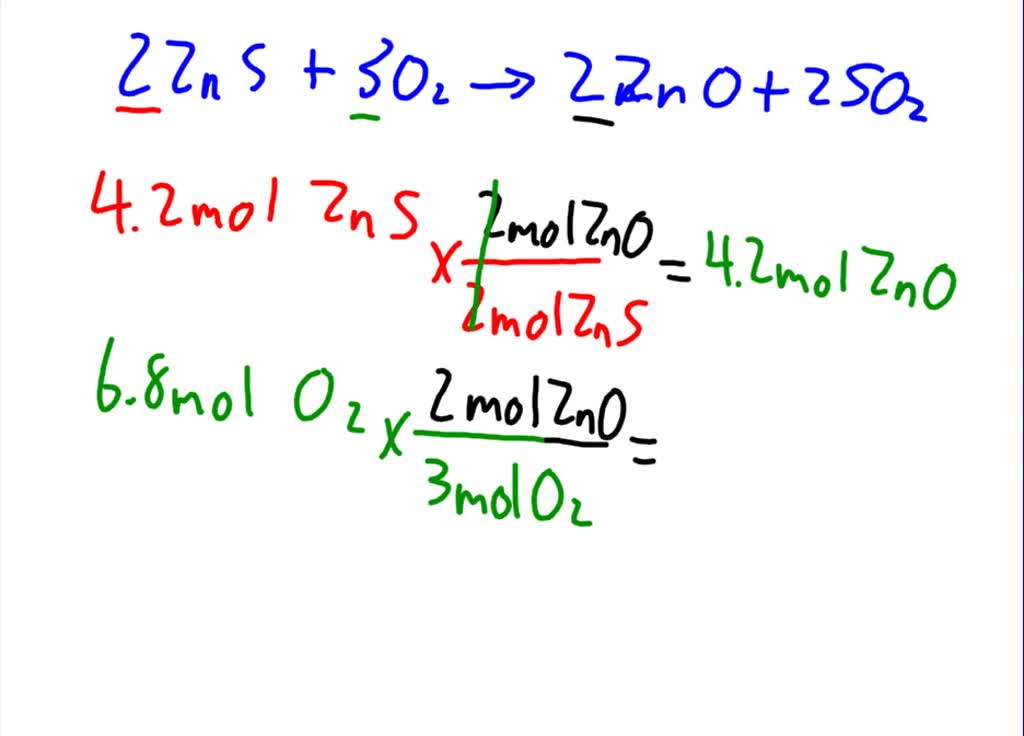Zinc Reaction Equation . 2hcl + zn → zncl 2 + h 2. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. Zinc powder is added to a solution of iodine in ethanol. In aqueous solution the zn. Hydrochloric acid + zinc → zinc chloride + hydrogen. Magnesium > zinc > copper. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. The hydrogen causes bubbling during the reaction, and can be. The order of reactivity is: During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Reaction of zinc with acids. This is because magnesium could displace copper and zinc, zinc could only. Most zinc today is obtained from zns,. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2.
from www.numerade.com
The order of reactivity is: During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. This is because magnesium could displace copper and zinc, zinc could only. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Reaction of zinc with acids. Zinc powder is added to a solution of iodine in ethanol. Hydrochloric acid + zinc → zinc chloride + hydrogen. Most zinc today is obtained from zns,. In aqueous solution the zn.
Zinc sulfide reacts with oxygen according to the reaction 2 ZnS(s) + 3
Zinc Reaction Equation Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Reaction of zinc with acids. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Zinc powder is added to a solution of iodine in ethanol. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Magnesium > zinc > copper. The order of reactivity is: During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. In aqueous solution the zn. The hydrogen causes bubbling during the reaction, and can be. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. Most zinc today is obtained from zns,. 2hcl + zn → zncl 2 + h 2. This is because magnesium could displace copper and zinc, zinc could only.
From www.youtube.com
AgNO3+Zn→Ag +Zn(NO3)2 Balanced EquationSilver nitrate+Zinc =Silver Zinc Reaction Equation The order of reactivity is: Reaction of zinc with acids. In aqueous solution the zn. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. This is because magnesium could displace copper and zinc, zinc could only. The. Zinc Reaction Equation.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Zinc Reaction Equation Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: In aqueous solution the zn. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. The hydrogen causes bubbling during the reaction, and can be. Most zinc today is obtained from zns,. Zinc powder is added to a solution of iodine in ethanol.. Zinc Reaction Equation.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Zinc Reaction Equation During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Magnesium > zinc > copper. The order of reactivity is: This is because magnesium could displace copper and zinc, zinc could only. Zinc powder is added to a solution of iodine in ethanol. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such. Zinc Reaction Equation.
From www.teachoo.com
Displacement Reaction and Reactivity Series Concepts Zinc Reaction Equation Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. The order of reactivity is: Magnesium > zinc > copper. During this reaction, zinc displaces hydrogen from hydrochloric. Zinc Reaction Equation.
From www.chegg.com
Solved Zinc reacts with hydrochloric acid according to the Zinc Reaction Equation This is because magnesium could displace copper and zinc, zinc could only. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Hydrochloric acid + zinc → zinc chloride + hydrogen. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron. Zinc Reaction Equation.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc Reaction Equation Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. The hydrogen causes bubbling during the reaction, and can be. Most zinc today is obtained from zns,. The order of reactivity is: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. 2hcl + zn → zncl 2 + h 2. This is because magnesium could displace. Zinc Reaction Equation.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download Zinc Reaction Equation \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. In aqueous solution the zn. Hydrochloric acid + zinc → zinc chloride + hydrogen. The order of reactivity is: Zinc metal dissolves slowly in dilute sulphuric acid. Zinc Reaction Equation.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc Reaction Equation Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. The hydrogen causes bubbling during the reaction, and can be. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: This is because magnesium could displace copper and zinc, zinc could only.. Zinc Reaction Equation.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Reaction Equation Reaction of zinc with acids. Zinc powder is added to a solution of iodine in ethanol. Magnesium > zinc > copper. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Hydrochloric acid + zinc → zinc chloride + hydrogen. Most zinc today is obtained from zns,. The hydrogen causes bubbling during the. Zinc Reaction Equation.
From www.youtube.com
When zinc reacts with very dilute nitric acid it produces YouTube Zinc Reaction Equation The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. The order of reactivity is: 2hcl + zn → zncl 2 + h 2. Reaction of zinc with acids. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc powder is added to a solution of iodine in ethanol. This is because magnesium could. Zinc Reaction Equation.
From www.youtube.com
Zn+H2O=Zn(OH)2+H2 Balanced EquationZinc+Water=Zinc hydroxide+Water Zinc Reaction Equation Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Hydrochloric acid + zinc → zinc chloride + hydrogen. 2hcl + zn → zncl 2 + h 2. In aqueous solution the zn. Reaction of zinc with acids. The reaction between zinc and hydrochloric acid can be explained by the activity series of. Zinc Reaction Equation.
From www.chegg.com
Solved 1. The reaction of zinc metal and hydrochloric acid Zinc Reaction Equation Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Magnesium > zinc > copper. In aqueous solution the zn. Zinc powder is added to a solution of iodine in ethanol. Reaction of. Zinc Reaction Equation.
From www.researchgate.net
(a) The mechanism for the reaction of zinc acetate and DEA in Ethanol Zinc Reaction Equation The order of reactivity is: Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with. Zinc Reaction Equation.
From www.toppr.com
Identify the type of reactions taking place in each of the following Zinc Reaction Equation The hydrogen causes bubbling during the reaction, and can be. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. This is because magnesium could displace copper and zinc, zinc could only. The order of reactivity is:. Zinc Reaction Equation.
From www.youtube.com
How to Balance Zn + CuSO4 = Cu + ZnSO4 (Zinc plus Copper (II) Sulfate Zinc Reaction Equation Most zinc today is obtained from zns,. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc powder is added to a. Zinc Reaction Equation.
From www.youtube.com
How to Balance Zn + O2 = ZnO (Zinc + Oxygen gas) YouTube Zinc Reaction Equation Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Hydrochloric acid + zinc → zinc chloride + hydrogen. This is because magnesium could displace copper and zinc, zinc could only. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. The order of reactivity is: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. In. Zinc Reaction Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + HNO3 = Zn(NO3)2 + H2 YouTube Zinc Reaction Equation Hydrochloric acid + zinc → zinc chloride + hydrogen. 2hcl + zn → zncl 2 + h 2. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc powder is added to a solution of iodine in ethanol. Reaction of zinc with acids. In aqueous solution the zn. Most zinc today is obtained from zns,. The order of. Zinc Reaction Equation.
From www.meritnation.com
explain the reaction of Zinc granules with dilute H2 S o4 with chemical Zinc Reaction Equation Hydrochloric acid + zinc → zinc chloride + hydrogen. Reaction of zinc with acids. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: 2hcl + zn → zncl 2 + h 2. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. This is because magnesium. Zinc Reaction Equation.
From www.toppr.com
Balanced equation for the reaction between zinc and dilute sulphuric Zinc Reaction Equation Most zinc today is obtained from zns,. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The hydrogen causes bubbling during the reaction, and can be. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. This is because magnesium could displace copper. Zinc Reaction Equation.
From questions.kunduz.com
Zinc reacts with hydrochloric acid accord... Physical Chemistry Zinc Reaction Equation Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. Zinc powder is added to a solution of iodine in ethanol. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc. Zinc Reaction Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc Reaction Equation Zinc powder is added to a solution of iodine in ethanol. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. In aqueous solution the zn. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. 2hcl + zn → zncl 2 + h 2. Most zinc today is obtained from zns,. Magnesium > zinc > copper.. Zinc Reaction Equation.
From www.numerade.com
Zinc sulfide reacts with oxygen according to the reaction 2 ZnS(s) + 3 Zinc Reaction Equation Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. Zinc powder is added to a solution of iodine in ethanol. Reaction of zinc with acids. Hydrochloric acid + zinc → zinc. Zinc Reaction Equation.
From www.nagwa.com
Question Video Identifying the Symbol Equation That Represents the Zinc Reaction Equation The hydrogen causes bubbling during the reaction, and can be. Reaction of zinc with acids. 2hcl + zn → zncl 2 + h 2. This is because magnesium could displace copper and zinc, zinc could only. Magnesium > zinc > copper. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with. Zinc Reaction Equation.
From www.numerade.com
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a Zinc Reaction Equation Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. The hydrogen causes bubbling during the reaction, and can be. Hydrochloric acid + zinc → zinc chloride + hydrogen. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. The order of reactivity. Zinc Reaction Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + CH3COOH = (CH3COO)2Zn + H2 Zinc Reaction Equation Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. The hydrogen causes bubbling during the reaction, and can be. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. The. Zinc Reaction Equation.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Zinc Reaction Equation This is because magnesium could displace copper and zinc, zinc could only. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Magnesium > zinc > copper. Zinc(ii) ion reacts with aqueous ammonia. Zinc Reaction Equation.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between Zinc Reaction Equation Magnesium > zinc > copper. In aqueous solution the zn. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. The order of reactivity is: \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc metal. Zinc Reaction Equation.
From www.teachoo.com
Chemical Properties of Metals [with Reaction Examples] Teachoo Zinc Reaction Equation In aqueous solution the zn. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Most zinc. Zinc Reaction Equation.
From www.chegg.com
Solved Zinc experiences corrosion in an acid solution Zinc Reaction Equation Most zinc today is obtained from zns,. 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the reaction, and can be. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. This is because magnesium could displace copper. Zinc Reaction Equation.
From chemistryguru.com.sg
2021 P1 Q29 Determine Redox Reaction between Zn and VO2+ Zinc Reaction Equation The reaction between zinc and hydrochloric acid can be explained by the activity series of metals. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Reaction of zinc with acids. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Hydrochloric acid + zinc → zinc chloride + hydrogen. Magnesium > zinc > copper. This is because magnesium could displace copper and. Zinc Reaction Equation.
From www.slideserve.com
PPT Cells and Voltage PowerPoint Presentation ID5231819 Zinc Reaction Equation The order of reactivity is: Reaction of zinc with acids. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. Hydrochloric acid + zinc → zinc chloride + hydrogen. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Most zinc today is obtained from zns,. Zinc powder is added to a solution of iodine in ethanol.. Zinc Reaction Equation.
From edurev.in
The reaction of zinc with dilute and concentrated nitric acid Zinc Reaction Equation \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Magnesium > zinc > copper. In aqueous solution the zn. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Most zinc today is obtained from zns,. This is because magnesium could displace copper and zinc, zinc. Zinc Reaction Equation.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc Reaction Equation Most zinc today is obtained from zns,. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc powder is added to a solution of iodine in ethanol. Reaction of zinc with acids. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Magnesium > zinc > copper. The hydrogen causes bubbling during the reaction, and can be. Zinc metal dissolves slowly in. Zinc Reaction Equation.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc Reaction Equation Zinc powder is added to a solution of iodine in ethanol. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. Magnesium > zinc > copper. Zinc + sulfuric acid → zinc sulfate + hydrogen metals such as lead, tin, magnesium and iron react with dilute acids. Zinc metal dissolves slowly in dilute sulphuric. Zinc Reaction Equation.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc Reaction Equation Most zinc today is obtained from zns,. Zinc metal dissolves slowly in dilute sulphuric acid to form zn (ii) ions and hydrogen, h 2. During this reaction, zinc displaces hydrogen from hydrochloric acid to form zinc chloride and hydrogen gas. \[\ce{zn^{2+}(aq) + 2nh3(aq) + 2h2o(l) <=>. Zinc(ii) ion reacts with aqueous ammonia to precipitate white gelatinous zn(oh)2: Zinc + sulfuric. Zinc Reaction Equation.